Why do patients hurt more in the morning than in the evening? Why do rheumatologists ask about morning stiffness and not evening stiffness? Why is it important in clinical trials to assess the effects of therapy at the same time point during the day?
The answers to all of these questions relate to the circadian rhythm. The term circadian rhythm refers to the 24-hour cycles in the physiological processes of living organisms that include, among others, the cycle of hormone levels or nerve activity. While circadian rhythms are a fundamental feature of the biology of animals, they are usually not considered as important determinants of disease, although their influence is profound.
In this article, we will address two issues: Why is the circadian rhythm relevant for the clinical practice in rheumatology? and How can the circadian rhythm influence the symptoms of my patient?
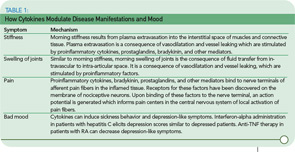
In considering symptoms of RA, it is remarkable that the improvement of morning stiffness induced by prednisolone after six months is similar in magnitude to the improvement of morning stiffness during a single day (see Figure 1, p. 15). Under both conditions—the clinical trial and the course of one day—an improvement of 40% to 50% can occur. Imagine, therefore, a situation in which an investigator in a clinical trial did not pay attention to the time point of examination and recorded improvement that reflected the diurnal variation as much as a sustained treatment effect.
Because patient symptoms can vary during the course of a day in clinical trials, assessment of symptoms can also vary depending on the time point of the visit. Patients often feel better later in the day. Should one therefore recommend an afternoon visit for the patient because the symptoms are less severe then? Indeed, many patients would prefer an afternoon visit because it is much easier for them to get up and around and travel to the clinic. Because symptomatology can have a pronounced diurnal cycle with a maximum in the morning, the underlying causal mechanisms are relevant for pathophysiology of rheumatic diseases, for clinical patient care, and for optimizing treatment strategies.
The Discovery of Circadian Rhythms
In the early 1970s, studies using brain-lesion techniques as well as metabolic and electrophysiological experiments indicated that, in mammals, there is a key structure in the brain which governs biological rhythms.1 As these studies showed, this central circadian oscillator is located in the hypothalamic suprachiasmatic nucleus (SCN) (see Figure 2, p. 16). The oscillator induces 24-hour cyclic changes of membrane potentials of neurons of the SCN.
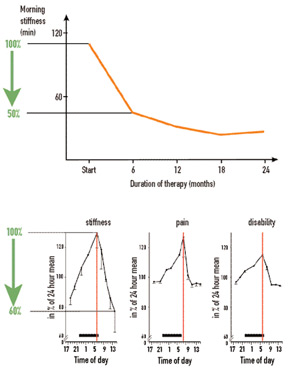
In the following years, links between the SCN and many centers in brain were discovered. Among others, the endocrine and sympathetic nervous centers are controlled by the SCN. Because the circadian rhythm is generated in the SCN of the hypothalamus, observations can provide new clues to understand neuroendocrine immune pathways relevant to rheumatic diseases. Because these aspects have been best elaborated in RA, the findings in this disease are discussed here. The mechanisms responsible for oscillation in suprachiasmatic neurons will not be considered but are reviewed elsewhere.2
Circadian Rhythms of Serum Cytokine and Hormone Production
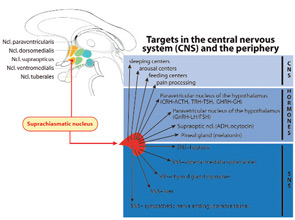
Because of the favorable effects of anti–tumor necrosis factor (TNF)–α and anti–interleukin (IL)-6 therapy in large clinical trials, rheumatologists recognized the central role of these proinflammatory cytokines in rheumatic diseases. These cytokines are also important in symptoms such as stiffness, joint swelling, pain, and mood (see Table 1, p. 15). An important question, therefore, is whether cytokines demonstrate a circadian oscillation. Indeed, both IL-6 and TNF-α display a circadian rhythm, with maximum levels in the early morning hours (see Figure 3, p. 16).2 In healthy subjects, the peak value of IL-6 is found at about 6 a.m. In patients with RA, the peak value of IL-6 appears at 7 a.m. Thus, a morning time shift of the peak value occurs with inflammatory disease. In healthy subjects, IL-6 serum levels are approximately 2–4 pg/ml whereas in patients with RA these levels are 20–40 pg/ml.
From these data, it is evident that the amplitude of the curve of cytokine production is higher and the curve is broadened in RA as compared to controls. In healthy subjects, serum levels of IL-6 usually decrease by 9 a.m. whereas, in RA patients, these levels can remain elevated until 11 a.m. The increased amplitude and the broadened curve in the morning hours are important for the morning symptoms. Interestingly, a circadian rhythm exists for the production of immunoglobulins in RA, as has been demonstrated for IgA–rheumatoid factor (peak at 8 a.m.).3 Furthermore, circulating immune complexes demonstrate a circadian rhythmicity in RA with a peak between 6 and 9 a.m.4 These findings demonstrate that two other important disease-relevant parameters display an elevation in the early morning hours in patients with RA.
In view of these observations, the key question is, What is driving the circadian oscillation of serum levels of cytokines?
Circadian Rhythm of Cortisol
The adrenal hormone cortisol is an endogenous steroid with anti-inflammatory actions. In the 1950s and 1960s, shortly after the discovery of cortisol, the circadian rhythm of this hormone was described. The cycle of the hypothalamic-pituitary-adrenal (HPA) axis has a maximum in the early morning hours at 8 a.m. and a nadir at midnight (see Figure 3, p. 16). Detailed analyses of the circadian curves of cytokines and cortisol have revealed a lag time between the cortisol rise in comparison to the increase of cytokine of approximately 60–120 min.5 From this study and a recent analysis of several independent investigations, it appears that morning cytokines can drive the increase of cortisol.2
Interestingly, the cortisol rhythm in healthy subjects does not differ from that of patients with RA when disease activity is relatively low to moderate. This similarity pertains to the period, the amplitude, and the time point of the minimum and peak of the cycle. Studies have shown, however, that this rhythm can be disturbed in patients with RA when disease activity is high, leading to a flattening of the hormone curve. Nevertheless, the serum levels of cortisol are similar in healthy subjects compared to patients with RA.
Although levels of cytokine ten times higher than normal should drive a stronger cortisol response, it is notable that the serum levels of cortisol are similar in healthy controls and patients with RA. This phenomenon is called inadequate secretion of cortisol in relation to inflammation. The reasons for this inadequacy relate in part to an inhibition of the HPA axis by chronically elevated cytokines. In other words, secreted cortisol is unable to dampen the proinflammatory response. As such, cytokine levels stay high.
What, then, is the factor that drives the nightly cytokine surge? At present, it is thought that the cortisol nadir at midnight and the parallel increase of proinflammatory hormones such as prolactin and melatonin drive the increase of nightly TNF-α and IL-6. These two cytokines drive cortisol, which finally dampens the cytokine surge, and so forth. The influence of one system on the other can be viewed as an infinite loop. Because the circadian rhythm is generated in the higher brain centers of the endocrine and nervous system (in the hypothalamus), the circadian rhythms of cytokines mirror the activity of neuroendocrine centers in the brain.
These findings are intriguing and provide a strong indication that neuroendocrine pathways influence disease-related pathophysiology. They also suggest new therapeutic options.
New Clues to Therapeutic Intervention
One would think that a therapeutic increase of circulating glucocorticoids should alleviate symptoms in patients with RA, and this has been demonstrated in Figure 1, Part A (left). These glucocorticoids are usually given after the patient awakes in the morning. One pioneering study demonstrated that administration of the same dose of prednisolone at 2 a.m. was significantly better than that dose given at 7:30 a.m. because it decreased morning stiffness, morning pain, disease activity assessed by the Lansbury index, and serum IL-6 after only five days of glucocorticoid treatment.6 These authors included two similar groups of patients with RA, who received 5.0–7.5 mg prednisolone either at 2 a.m. (therapeutic group I, n=13) or at 7:30 a.m. (therapeutic group II, n=13). Baseline values of the two therapeutic groups were very similar. No placebo group or a cross-over design was included, but the results are quite remarkable.6
Although this study did not attract widespread interest or make the daily press, it stimulated pharmaceutical companies to develop time-release prednisone tablets. Recent data of a double-blind, placebo-controlled, randomized study in hundreds of patients with RA demonstrated a marked effect on morning stiffness and serum IL-6.7 The question appears to be why immunosuppressive treatment with glucocorticoids can inhibit pro-inflammatory sequelae better when given at an early time point, 2 a.m. (on the increasing flank of TNF-α release), than a later one, 7:30 a.m. (on the falling flank of TNF-α release) (see Figure 4, p. 16).
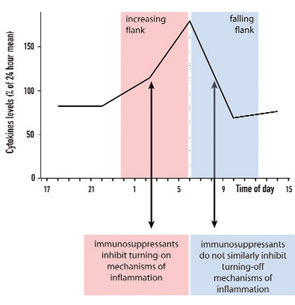
These observations are very important in understanding anti-inflammatory counter-regulation of immune responses. It is has been demonstrated that glucocorticoids induce the transcription of the IκBα gene, which results in an increased rate of IκBα protein synthesis and inhibition of proinflammatory NF-κB effects.8 Other studies have shown that glucocorticoids can interfere with the transcriptional activation potential of DNA-bound NF-κB complexes leading to anti-inflammatory effects.9 These effects appear very early in the turning-on phase of a proinflammatory response (see Figure 4, p. 16). We hypothesize that the turning-on phase of a proinflammatory reaction is much more vulnerable to immunosuppressants than the turning-off phase. This suggests that regulation of an important proinflammatory factor such as TNF-α must occur very early; otherwise an overwhelming secretion of this harmful cytokine will occur.
In view of the circadian rhythm and the impact of the timing of immunosuppressive administration on efficacy, it would be of great interest to explore timed-release forms of cytokine neutralizers (on the basis of small molecules) or hormonal antagonists of melatonin, for example, as therapeutic modalities. Reformulating old drugs in new drug delivery forms could lead to optimized drug release patterns with improved immunosuppressant activities. Stronger inhibition of nighttime proinflammatory cytokines such as TNF-α and IL-6 could reduce RA-related symptoms in the early morning—and possibly, over a long time, to less RA-related co-morbidities such as joint disease, cardiovascular disease, osteoporosis, depression, and sleep disturbances.
Dr. Straub is professor of experimental rheumatology and neuroendocrino-immunology in the department of internal medicine I at the University Hospital in Regensburg, Germany. Dr. Cutolo is professor in the research laboratory and academic unit of clinical rheumatology in the department of internal medicine at the University of Genoa in Italy.
References
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201-206.
- Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis Rheum. 2007;56:399-408.
- Olsen NJ, Brooks RH, Furst D. Variability of immunologic and clinical features in patients with rheumatoid arthritis studied over 24 hours. J Rheumatol. 1993;20:940-943.
- Petersen I, Baatrup G, Brandslund I, et al. Circadian and diurnal variation of circulating immune complexes, complement-mediated solubilization, and the complement split product C3d in rheumatoid arthritis. Scand J Rheumatol. 1986;15:113-118.
- Crofford LJ, Kalogeras KT, Mastorakos G, et al. Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: Failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 1997; 82:1279-1283.
- Arvidson NG, Gudbjornsson B, Larsson A, Hallgren R. The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis. 1997;56:27-31.
- Buttgereit F, Doering G, Schaeffler A, Szechninski J, Alten R. New modified-release (MR) tablet formulation of prednisone significantly reduces duration of morning stiffness compared to standard prednisone in subjects with rheumatoid arthritis (Abstract). Ann Rheum Dis. 2007;66 (Suppl. II):52.
- Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283-286.
- Ray KP, Farrow S, Daly M, Talabot F, Searle N. Induction of the E-selectin promoter by interleukin 1 and tumour necrosis factor alpha, and inhibition by glucocorticoids. Biochem J. 1997;328(Pt 2):707-715.
- Zeidler H, Rau R, Steinfeld P. Efficacy and safety of low dose prednisolone in early rheumatoid arthritis (RA) (Abstract). Arthritis Rheum. 1999;42(Suppl. 9):271.
- Kees MG, Pongratz G, Kees F, Schölmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol. 2003;145:77-85.