Lyme disease has been a difficult journey.
—Allen Steere MD
In 1975, Polly Murray, an artist residing in the idyllic town of Old Lyme, Conn., was clearly worried about the health of her four young children.1 Recently they had all developed skin rashes and flu-like illnesses. One son, Todd, had been diagnosed with juvenile rheumatoid arthritis (JRA) and another son, Alex, had developed an unexplained stiff and swollen knee. Murray was not reassured by the pediatrician’s assessment that there was absolutely no link between their illnesses. Nor were her fears allayed by the discovery that twelve children in Lyme had recently been diagnosed with JRA. There were no obvious explanations for this outbreak. Some people speculated that a nearby nuclear power plant was to blame. Others ascribed it to contaminants present in the town’s drinking water supply.
I have heard from reliable sources that Murray contacted several Boston-area teaching hospitals seeking their help, but to no avail. She found a more receptive audience in Connecticut, where an epidemiologist working in the State Department of Health put her in contact with Allen Steere, MD, then a first-year rheumatology fellow at the Yale School of Medicine in New Haven, Conn. Dr. Steere, whose previous training had been in the field of epidemiology, paid a visit to Lyme, a wooded hamlet of 5,000 residents nestled at the mouth of the Connecticut River. He was intrigued. A preliminary surveillance study of three contiguous towns—Lyme, Old Lyme, and East Haddam—identified 39 children and 12 adults with some form of inflammatory arthritis.2 Most of the patients resided in heavily wooded, sparsely settled areas and there appeared to be a peak occurrence of the illness in the summer months. What might be responsible for this outbreak? The patients’ physical examination findings and laboratory test results did not point to a specific cause. The breakthrough clue came after a meticulous review of the patients’ histories, when Dr. Steere discovered that one-quarter of them had noticed a rash in the shape of a bull’s-eye pattern develop a few weeks before the onset of the arthritis. Olé!
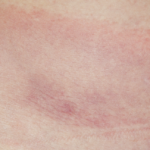
Bull’s Eye
The history of the bull’s-eye rash brings us back to the early years of the twentieth century when a Swedish dermatologist, Dr. Arvid Afzelius, presented a paper to the Dermatologic Society of Stockholm that described a patient with an expanding skin lesion, which he termed erythema migrans. Paradoxically, this rash, which faded within a matter of weeks, was renamed erythema chronicum migrans. A dozen years later, Dr. Afzelius, who studied under the famed Hungarian physician and dermatologist Moritz Kaposi, presented a second report on a series of six patients with this rash:3
“The clinical picture is very characteristic. The illness begins with one (and always only one) fairly small round blotch. This spreads outward so that the outer periphery forms a small 1 to 2 cm wide ring. This ring gradually widens, while the center fades little by little until it assumes a wholly natural skin color, sometimes with a mild cyanotic tone. The wider the ring extends peripherally, the paler and less distinct it becomes until at last, after several weeks or at most after a few months, it disappears entirely.”
Not everyone was impressed. A French dermatologist, Dr. Jacques Strandberg, described the case of a woman who developed a similar lesion that began on her upper arm. Five months later, it had extended “across the breast and could only with difficulty be hidden by the application of powder when the patient wore a decollete dress.”4 Nonetheless, he concluded that, “the disease is of no importance for its own sake and is rather to be looked on as a freak.” Perhaps Dr. Strandberg was too focused on fashion rather than biology! In contrast, Dr. Afzelius was not sure about the etiology of the rash, but speculated that it was “introduced by the bite of a tick (Ixodes reduvius) or another insect.”3 This concept lay dormant for the next half century.
Fast-forward to the summer of 1977. Dr. Steere, armed with the knowledge that residents of a wooded area were developing bull’s-eye rashes and arthritis, telephoned Willy Burgdorfer MD, PhD, a specialist in the field of tickborne diseases at the Rocky Mountain National Laboratories in Hamilton, Mont., to inquire about how to evaluate ticks for secondarily acquired microorganisms. After all, ticks were known as vectors for a host of illnesses ranging from babesiosis to Q fever to Rocky Mountain spotted fever.
Over the next few years, Dr. Burgdorfer collaborated with Dr. Steere and others in an effort to identify the microorganism that might be the causative agent of this newly described illness, Lyme disease. First, he attempted to recover rickettsiae from the eastern deer tick, Ixodes dammini, (named after its discoverer, a pathologist at my hospital, Gustave Dammin, MD) garnered from Shelter Island, N.Y., a well-known endemic locus of Lyme arthritis. Yet, when he examined Giemsa-stained smears taken from the midgut diverticula of two such ticks, he did not find the expected rickettsial microorganisms, but instead discovered the presence of, “rather long, irregularly coiled spirochetes.”5 Indirect immunofluorescence studies revealed that antibodies extracted from the serum of Lyme disease–infected patients reacted positively with the spirochete, while serum from control patients did not, thereby confirming the relationship between the tick-derived spirochete and Lyme disease. The final link to Dr. Afzelius’ bull’s-eye lesion had been formed. Keeping with tradition, the organism was named Borrelia burgdorferi.
The Rheumatologist’s Perspective
For those of us who practice in endemic areas, seeing and diagnosing Lyme disease should be fairly straightforward. Most patients present with either a mono- or oligoarticular large-joint synovitis that has a predilection for involving the knee. It should rarely be confused with rheumatoid arthritis. However, many patients are mistakenly under the impression that B. burgdorferi infection may be the cause of their chronic polyarticular joint achiness. In the earliest stage of Lyme disease, one may develop a flu-like illness with polyarthralgias along with the rash, but these symptoms subside within a matter of days.
One fascinating aspect of Lyme arthritis has been its evolution from being an infectious disease to becoming an autoimmune disorder in some individuals. This transition was well summarized in a recent study led by Dr. Steere, currently professor of medicine at the Harvard Medical School in Boston.6 As evidence against the persistent infection hypothesis, polymerase chain reaction and culture results of synovectomy specimens obtained following antibiotic use have been uniformly negative. In addition, relapse of infection has not been observed with the use of disease-modifying antirheumatic drugs such as methotrexate or anti–tumor necrosis factor inhibitors after antibiotic therapy.
Contrary to what might be expected with retained spirochetal antigens, T- and B-cell responses to B. burgdorferi decline to a similar degree in patients with refractory arthritis and in those with responsive arthritis. Yet, levels of inflammatory mediators in synovial fluid, such as interferon-gamma, remain high or even increase in patients with refractory arthritis during the postantibiotic period. Although these inflammatory mediators are initially important for the eradication of the spirochete, the inability to downregulate their expression in these individuals likely contributes to immune dysregulation and persistence of the arthritis.
Additional evidence in support of the autoimmunity hypothesis is the observation that specific HLA–DR alleles, particularly the DRB1*0101 or 0401 alleles, are the greatest-known genetic risk factor for developing an antibiotic refractory arthritis. Similar to other chronic inflammatory arthritides, HLA–DR molecules in antibiotic refractory Lyme arthritis are intensely expressed in inflamed synovium. Might this transformation from infectious illness to an autoimmune disorder serve as a paradigm for other inflammatory disorders?
A World of Controversy
Infection with B. burgdorferi can extend beyond the joints. Aside from inflaming heart tissues, this spirochete is also capable of finding its way to the central nervous system (CNS). Certainly this is the case for a fellow spirochete, Treponema pallidum. Back in the old days, medical students and interns were taught that the first step in establishing the diagnosis of neurosyphilis was to confirm that the patient had actually been infected with T. pallidum, since one could not have neurosyphilis without first having syphilis.
Paradoxically, with regards to Lyme disease, some practitioners seem to ignore this rule.7 In their view, the lack of antibody positivity does not preclude the diagnosis of Lyme disease. Furthermore, many of them reject the criteria for diagnosing Lyme disease proposed by the Centers for Disease Control and Prevention or by the Infectious Diseases Society of America (IDSA). In its place, they have coined the term, “chronic Lyme disease” to explain a variety of vague symptoms such as chronic fatigue and cognitive dysfunction that plague many of their patients. One of the chronic Lyme advocacy websites lists 25 possible symptoms of Lyme disease, including testicular or pelvic pain, back pain, sleep disturbances, and mood swings.8
Although the vast majority of individuals given the diagnosis of chronic Lyme disease do not fulfill the IDSA criteria that are used to diagnose Lyme disease, many self-proclaimed “Lyme literate” physicians liberally prescribe nostrums, including prolonged antimicrobial therapies, in a belief that this approach can eradicate suspected infection and cure the patient of what ails them. Their patients and supporters have created vocal advocacy organizations and established some strong alliances with some politicians.
One relationship in particular stands out. In November 2006, the Connecticut Attorney General (and currently the senior U.S. Senator from Connecticut) Richard Blumenthal initiated an investigation to determine whether the IDSA violated antitrust laws in the promulgation of its 2006 Lyme disease guidelines. He leveled some serious accusations against the IDSA. “The IDSA’s 2006 Lyme disease guideline panel undercut its credibility by allowing individuals with financial interests—in drug companies, Lyme disease diagnostic tests, patents, and consulting arrangements with insurance companies—to exclude divergent medical evidence and opinion.”9 The IDSA maintained that it had developed the 2006 Lyme disease guidelines on the basis of a proper review of the medical or scientific studies and evidence by a panel of experts in the prevention, diagnosis, and treatment of Lyme disease.
A state attorney general wields a big stick. During his tenure, Blumenthal sued tobacco companies, polluters, Internet pornographers, and other violators of the public trust. Attacking the credibility and professionalism of a medical specialty society made little sense and, in my view, tarnished his image. Perhaps he had some misgivings about the lawsuit because, 18 months after filing it, Blumenthal accepted a proposal from the IDSA to convene an independent panel of medical experts to conduct a one-time review of their guidelines.10 The review panel members, vetted by an ombudsman for potential conflicts of interest, reviewed the entirety of the 2006 guidelines, with particular attention to the recommendations devoted to post–Lyme disease syndromes. After multiple meetings, a public hearing, and extensive review of research and other information, the panel concluded that the recommendations contained in the 2006 guidelines were medically and scientifically justified on the basis of all of the available evidence and that no changes to the guidelines were necessary. Case closed? Not really. Perusing some of the vitriolic commentary on the Internet about the IDSA guidelines will dispel you of that myth!
The Tick and The Iceman
Though the uproar over Lyme disease is a 20th century phenomenon, the origins of the illness go back many centuries. The Tyrolean Iceman, a 5,300-year-old Copper Age individual, whose remains were remarkably preserved, was discovered in 1991 on the Tisenjoch Pass in the Italian Alps.11 An analysis of his genome found genetic sequences that contained approximately 60% of the B. burgdorferi genome, making him the earliest confirmed case of Lyme disease. The Iceman was found buried in a snow bank, having met a violent death. An arrowhead was lodged within the soft tissue of his left shoulder, causing substantial damage to the left subclavian artery. Bull’s eye!
Dr. Helfgott is physician editor of The Rheumatologist and associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School in Boston.
References
- Daley B. Drawing the lines in the Lyme disease battle. Boston Globe. Published June 1, 2013. Available at www.bostonglobe.com/metro/2013/06/01/lyme-disease-rise-and-controversy-over-how-sick-makes-patients/OT4rCTy9qRYh25GsTocBhL/story.html. Accessed July 12, 2013.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis. An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20:7-17.
- Afzelius A. Erythema chronicum migrans. Acta Derm Venereol. 1921;2:120-125.
- Strandberg I. Regarding an unusual form of migratory erytbema caused by tick bites. Acta Dermatovenereol. 1920;1:422-427.
- Burgdorfer W. Discovery of the Lyme disease spirochete and its relation to tick vectors. Yale J Biol Med. 1984;57:515-520.
- Drouin EE, Seward RJ, Strle K, et al. A novel human autoantigen, endothelial cell growth factor, is a target of T and B Cell responses in patients with Lyme disease. Arthritis Rheum. 2013;65:186-196.
- Feder HM, Johnson BJB, O’Connell S, et al. A critical appraisal of “chronic Lyme disease.” N Engl J Med. 2007;357:1422-1430.
- International Lyme and Associated Diseases Society. International Lyme and associated disease treatment guidelines. Available at www.ilads.org/lyme_disease/treatment_guidelines_summary.html. Accessed July 12, 2013.
- Connecticut Office of the Attorney General. Attorney General’s investigation reveals flawed Lyme disease guideline process, IDSA agrees to reassess guidelines, install independent arbiter [Press Release]. Published May 1, 2008. Available at www.ct.gov/ag/cwp/view.asp?a=2795&q=414284. Accessed July 12, 2013.
- Lantos, PM, Charini WA, Medoff G, et al. Final report of the Lyme disease review panel of the Infectious Diseases Society of America. Clin Infect Dis. 2010;51:1-5.
- Keller A, Graefen A, Ball M, et al. New insights into the Tyrolean Iceman’s origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698.