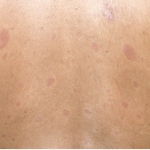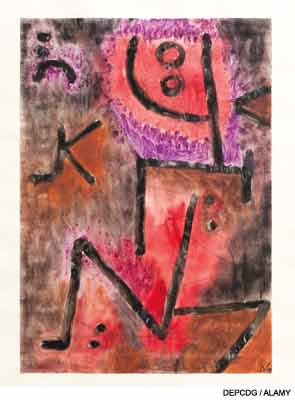

In its more aggravated forms, diffuse scleroderma is one of the most terrible of all human ills. Like Tithonus, to wither slowly, and like him to be beaten down and marred and wasted until one is literally a mummy, encased in an ever shrinking, slowly contracting skin of steel, is a fate not pictured in any tragedy, ancient or modern.
—Sir William Osler
The pain passes but the beauty remains.
—Pierre-Auguste Renoir
The great 20th century Swiss painter Paul Klee might have benefitted from the care of a rheumatologist. A complex and enigmatic artist, he left behind a staggering 10,000 pieces of art at the time of his death in 1940. He constantly experimented with new techniques and styles, drawing squares, triangles, or domes using sun-splashed colors. He believed that the role of abstract art was to create the reality behind visible things.1
In 1933, dark clouds were amassing over Germany. Klee refused to declare his loyalty to the newly installed Nazi regime and was dismissed from his job. The persecution intensified, and his works were removed from private and public German collections. Seventeen of his paintings, along with others produced by Pablo Picasso, Edvard Munch, and Marc Chagall, were included in Joseph Goebbels’ infamous Entartete Kunst (Degenerate Art) show. The exhibition, which was viewed by millions of Germans, was designed to ridicule and denigrate creative art that did not uphold “correct” National Socialist virtues. Most of the exhibit was burned in the courtyard of a Berlin fire station, and some pieces were auctioned off to the highest bidders.2
It was around this time that Klee, who had fled to Bern, Switzerland, became ill. He developed severe fatigue and a skin rash that his physicians speculated was due to measles. However, this would have been a rather unlikely diagnosis in a 56-year-old male. An alternative interpretation has been offered by an expert on Klee, our colleague, John Varga, MD, the John and Nancy Hughes Distinguished Professor in rheumatology at Northwestern University in Chicago. He hypothesized that Klee’s facial rash was caused by an eruption of telangiectasia, the earliest sign of what became a full-blown case of scleroderma.2 Within months, this avid hiker experienced exertional dyspnea and worsening dysphagia that restricted his diet to liquids. In his letters, he described having arthritic pain in his hands, making it difficult to hold a paintbrush. As Klee’s health declined, his friends, Pablo Picasso and Georges Braque, came to pay homage. Despite being given the diagnosis of scleroderma, Paul Klee was not done painting.
A Most Mysterious Ailment
Scleroderma is a most vexing illness. First, there is the issue of what to call this disease.
Two of our former colleagues, whose names are synonymous with scleroderma, Thomas Benedek, MD, professor emeritus, and the late Gerald Rodnan, MD, both of the University of Pittsburgh, catalogued more than twenty different terms that have been used to describe this condition.3 Recently, there has been a shift towards adopting the more comprehensive term, systemic sclerosis (SSc), which includes the potentially devastating aspects of the illness that are not so readily visible. Second, the absence of a sensitive diagnostic test has made the diagnosis of early disease quite challenging. More commonly, patients present with advanced features of fibrosis, such as finger contractures and lung disease. Managing their illness can be highly challenging, since the course of SSc is often hard to predict. Third, it is not exactly clear how SSc begins. According to the 2013 ACR/European League Against Rheumatism criteria, the pathogenesis of SSc is characterized by three features: a small vessel vasculopathy, the production of autoantibodies, and the development of fibroblast dysfunction leading to increased deposition of extracellular matrix.4 Yet, how do these pathways interact with one another? Which one precedes the others? Does the universal finding of Raynaud’s phenomenon in patients with SSc suggest a vascular-based etiology? Why does the disease follow an implacable course in some individuals while remaining static in others?
Unlike other rheumatologic diseases, the therapeutic armamentarium for SSc is fairly limited. It is not clear whether our standard immunosuppressive therapies have any useful role to play. At present, mitigating fibrosis remains out of therapeutic reach. Instead, drugs that target vascular structures, such as prostanoids, endothelin-1 receptor antagonists, angiotensin-converting enzyme inhibitors, and inhibitors of phosphophodiesterase-5 (PDE5)may provide benefit to selected patients. For example, the selective inhibition of tyrosine kinase by imatinib interferes with the signaling of both platelet-derived growth factor (PDGF) and transforming growth factor (TGF) β, two pivotal mediators of the fibrotic process in SSc. After showing some promise in early studies, subsequent larger trials have been disappointing.
A new approach that aims to identify the key processes responsible for tissue fibrosis has focused on a well-known signal transduction pathway that has been known to play an important role in embryonic development and carcinogenesis. This highly conserved wingless-related integration site pathway, better known as Wnt signaling, consists of proteins with some impish names such as Frizzled and Dishevelled. A recent study demonstrated that the canonical Wnt pathway is activated in fibrotic diseases and potently stimulates fibroblast activation and tissue fibrosis.5 TGF-β signaling decreases the expression of Dickkopf-1 (Dkk-1) and activates the Wnt pathway. In fact, inhibition of canonical Wnt signaling by overexpression of Dkk-1 significantly reduced the profibrotic effects of TGF-β, demonstrating that the interaction of the canonical Wnt pathway and TGF-β may play a key role in the pathogenesis of fibrotic diseases. Could a disease as difficult to treat as SSc be tamed via the overproduction of Dkk-1, whose name, in German, means stubborn or obstinate?
What Can We Learn From Other Fibrosing Disorders?
Given the rarity of Ssc (for example, rheumatoid arthritis is about 20-fold more common), it may be of interest to see whether other fibrotic disorders can provide some clues into the pathogenesis of this disease. In the spring of 1981, an explosive outbreak of pneumonitis occurred in Spain, focused at first in the vicinity of Madrid and then extending to provincial areas, especially to the northwest. Patients typically presented with cough, dyspnea, pleuritic chest pain, headache, fever, and bilateral pulmonary infiltrates on imaging studies.6 Some patients developed skin thickening and joint contractures, Raynaud’s phenomenon, and debilitating neuropathies. Initially, the epidemic was attributed to infectious causes, but shortly after the outbreak, a strong association with food oils sold as olive oil but containing a high proportion of rapeseed oil began to be recognized. These suspect oils were identifiable as being inexpensive, sold in unlabeled five-liter plastic containers, and usually acquired from itinerant salesmen. Epidemiologic studies confirmed that all patients had ingested this tainted oil. Though the precise cause of the epidemic is not known, it most likely involved the adulteration of a particular batch or batches of rapeseed oil. Denatured with 2% aniline and intended for industrial use, rapeseed oil was regularly imported into Spain. Its low cost had encouraged chemical denaturation via the removal of aniline, with subsequent clandestine distribution for food use, primarily among poorer populations. Fortunately, the toxic oil syndrome has never been seen again.
At the end of the decade, in October 1989, the New Mexico Department of Health and Environment was notified of three patients with severe myalgia and peripheral eosinophilia. All three had been taking oral preparations of the amino acid L-tryptophan.7 Even though the patients had undergone extensive clinical evaluation and testing, their illnesses were not consistent with any known diagnostic entity. Public announcement of the cluster quickly led to reports of similar cases. Within one month, more than 30 additional cases were identified in New Mexico and about half of these patients had been hospitalized. Aside from a marked eosinophilia, most of them developed incapacitating myalgias, hence the term eosinophilia–myalgia syndrome (EMS) was coined.
Investigators analyzed a variety of predisposing factors, such as the use of different vitamins, other health foods or raw food products, medications, and water sources. They concluded that all of the affected patients were found to have recently ingested L-tryptophan–containing products. Subsequent studies confirmed that a change in the manufacturing protocol at one chemical plant in Japan accounted for most of the contaminated L-tryptophan supplements associated with the development of EMS. It was a dreadful illness. Aside from the incapacitating myalgias, which were only partially responsive to treatment with corticosteroids, some patients went on to develop a disabling, widespread fasciitis and an often fatal form of pulmonary fibrosis.
My single encounter with EMS occurred one month after the Morbidity and Mortality Weekly Report had reported these first cases. Margaret was a 50-year-old woman who, on the advice of her psychiatrist, had started using L-tryptophan as a sleep supplement. He believed that this was a safer therapeutic alternative than having her continue taking diazepam to help her sleep. Sadly, her demise is best depicted by Dr. Osler’s quotation about scleroderma and mummies. The fatal blow came when Margaret developed a marked axonal neuropathy, resulting in a rapidly ascending polyneuropathy with the development of a near-total quadriplegia. Her tragic battle with EMS finally came to an end.
Recently, with the advent of new imaging techniques, we began witnessing a new disorder, nephrogenic systemic fibrosis (NSF). This generalized fibrotic disease developed in some patients with renal insufficiency following exposure to gadolinium-based contrast agents that were used to enhance magnetic resonance imaging. Clinically, NSF shares many common features with SSc, including severe and usually progressive skin induration, incapacitating joint flexion contractures, and fibrotic involvement of the lungs, heart, and other organs. Recent evidence suggests that exposure to gadolinium-based contrast agents results in the increased production and expression of numerous proinflammatory and profibrotic cytokines, chemokines, and growth factors.8
(For additional information, please refer to “Gadolinium Compounds Provide Insights into Pathogenesis of Fibrosing Diseases” at www.The-Rheumatologist.org.)
EMS, toxic oil syndrome, NFD, and other conditions including eosinophilic fasciitis and graft-versus-host disease can all mimic SSc. Unfortunately, our current understanding of the pathogenesis of these disorders has not led to a deeper understanding of SSc. The mystery remains unsolved.
A Painter’s Legacy
How did Paul Klee’s illness influence his art? In the year that he was diagnosed with SSc, he produced just 25 works, but three years later this number mushroomed to more than 1,200 pieces. What about the nature of his final years? According to Varga, Klee’s creative genius was fundamentally shaped by his illness.2 The lightheartedness and wit of his early works gave way to introspection and despair. Abstract figures were painted with simple, heavy black lines. Some even seemed to be childish or primitive. He abandoned painting intricate, small-scale compositions for much larger pieces. In some of his later art (“The Mask,” 1940, and “Captive,” 1940), the viewer can witness some of the profound changes in Klee’s personal appearance and his sense of being trapped inside a prison. Though Klee was dying and the world around him was collapsing amid the chaos of the Second World War, he found a way to persevere. Like Tithonus, “he withered slowly,” but unlike him, he refused “to be beaten down and marred and wasted.”
Dr. Helfgott is physician editor of The Rheumatologist and associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School in Boston.
References
- Cole TB. The cover. JAMA. 2013;309:1562.
- Varga, J. Illness and art: The legacy of Paul Klee. Current Opin Rheumatol. 2004;16:714-717.
- Rodnan GP, Benedek TG. An historical account of the study of progressive systemic sclerosis (diffuse scleroderma). Ann Intern Med. 1962:57;307-319.
- Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis. Arthritis Rheum. 2013:65;2737-2747.
- Akhmetshina A, Palumbo K, Dees C, Bergmann C. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735.
- Kilbourne EM, Riagu-Perez JG, Heath CW, Zack MM. Clinical epidemiology of toxic-oil syndrome. N Engl J Med. 1983;309:1408-1414.
- Epidemiologic notes and reports eosinophilia-myalgia syndrome—New Mexico. MMWR. 1989:38; 765-767.
- Wermuth PJ, Jimenez SA. Gadolinium compounds signaling through TLR4 and TLR7 in normal human macrophages: Establishment of a proinflammatory phenotype and implications for the pathogenesis of nephrogenic systemic fibrosis. J Immunol. 2012; 189:318-327.