RheumMadness is an online tournament in which a bracket of teams, representing key learning concepts in rheumatology, compete against each other in a series of head-to-head matchups, much like basketball teams in the NCAA’s March Madness.
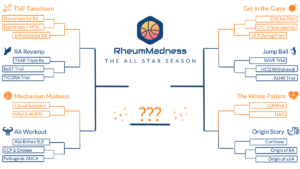
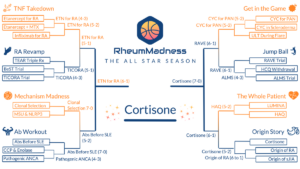
The 2023 tournament theme was The All Star Season. Each team represented one all star article competing to be named the most important and transformational article ever written in the field of rheumatology. Here, we feature the scouting reports from this year’s Final Four:
- Cortisone, by the University of Colorado Fellowship Program—the winner
- Etanercept for RA, by the Ohio State Fellowship Program
- Autoantibodies Before SLE, by the Massachusetts General Hospital Fellowship Program
- RAVE Trial, by the Wake Forest Fellowship Program
To see all the scouting reports, visit https://sites.duke.edu/rheummadness. RheumMadness is supported by a Clinician Scholar Educator Award from the Rheumatology Research Foundation and modeled after the NephMadness tournament in nephrology.
Rheum on the Rocks
Origins of corticosteroid treatment
BY SMARIKA SAPKOTA, MD, DAVID DEFRANCISCO, MD, & JASON KOLFENBACH, MD
Base Article
Hench PS, Kendall EC, Slocumb CH, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949 Apr 13;24(8):181–197.1
Team Overview
Philip Hench and colleagues published their landmark study in 1949, during an era in which knowledge about the pathologic underpinnings of rheumatoid arthritis (RA) was limited, as were treatment options. Patients at that time were treated with aspirin (first distributed by Bayer in 1899) or, much less commonly, gold or sulfonamide compounds (1930s), often with limited success.2,3 The initial use of sulfonamide compounds, such as sulfasalazine, as well as such medications as d-penicillamine (1950s), reflected a prevailing hypothesis that RA was a microbial-driven disease.
Hench et al. demonstrated remarkable clinical and laboratory improvements in RA disease control through administration of their “Compound E” (17-hydroxy-11-dehydrocorticosterone) to 14 patients with severe RA. This study was critical to the field of rheumatology not only for the discovery of an effective therapeutic agent (i.e., cortisone), but also because it supported a biochemical basis of disease rather than a microbial origin.
Prior to publication of this study, Hench had studied the beneficial effects of pregnancy and jaundice on RA. The improved disease activity he observed in these settings was a novel finding because RA was previously thought to be an irreversible disease. Based on his observations, he sought a common biochemical denominator between pregnancy and jaundice that could lead to a potential therapeutic intervention for RA. After a series of trial-and-error experiments, the team settled on an adrenal hormone as the most likely “anti-rheumatic substance X,” and on Sept. 21, 1948, began treating their first patient with Compound E.
This study describes the subjective and objective responses of 14 patients following treatment with Compound E. Improvement was noted in muscle and joint pain, functional status, appetite, strength and overall sense of well-being—including the first description of steroid-associated euphoria. In addition, reductions in erythrocyte sedimentation rate and serum gamma globulin were reported, as well as improvement in disease-associated anemia. The biologic effect of Compound E was further supported by studies of drug withdrawal and subsequent re-institution upon disease flare.
Impact on Rheumatology
This landmark study provided the first evidence that corticosteroids could significantly improve the lives of patients suffering from RA, and its results suggested an alternative to the microbial theory of disease pathogenesis. This latter discovery shaped research in the decades to follow, helping steer the field away from viewing RA as primarily the result of an infectious agent and shifting the focus toward therapeutics without anti-microbial properties. The experiments described in this study also relied upon collaboration with industry partners (i.e., Merck and Co.), a foreshadowing of the academic-industry relationship that drives many drug discoveries today.
Following the improvement seen in patients with RA, Hench et al. then began to administer Compound E to lupus patients, with “encouraging results,” and described a patient with myasthenia gravis who improved following treatment with adrenocorticotropic hormone. These point to the widespread applicability of corticosteroids in inflammatory/autoimmune disease.
Chances in the Tournament
Do we love this study? Let us count the ways: the discovery of the therapeutic potential of steroids, evidence of a biochemical basis of RA, initial descriptions of steroid-related side effects, and application to other diseases such as lupus and myasthenia gravis. Oh, and yes, the authors were awarded the Nobel Prize (the following year!) as a result of their decades-long work in this area.
While we believe this study is a clear favorite to win the tournament, it may also be viewed by some readers as a blue blood that needs to step aside and make way for new therapies. Just as many fans have become tired of seeing Duke basketball play in the past 25 NCAA tournaments or Southeastern Conference football teams annually winning the championship game, some may feel that prednisone has already had its day in the sun.
It may also be tempting to overlook this study due to the side effects associated with corticosteroids, as well as numerous studies investigating the ability to reduce our dependence on them.4-6 Even so, it’s important to recognize that all medications have side effects, and as with any drug, medication risk can be mitigated by a cautious, conscientious prescriber and balanced against the potential benefit. With this said, let’s recognize the many positives this precious gift provides to our patients with rheumatic disease and give credit where it’s due.
So the next time you are consulted on a patient with diffuse alveolar hemorrhage and a pulmonary-renal syndrome in the intensive care unit, think of the value of corticosteroids, and the impact their discovery has had on the field of medicine.
All authors are part of the Rheumatology Fellowship Program at the University of Colorado, Anschutz Medical Campus, Aurora.
References
- Hench PS, Kendall EC, Slocumb CH, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949 Apr 13; 24(8):181–197.
- Sneader W. The discovery of aspirin: A reappraisal. BMJ. 2000 Dec 23;321(7276):1591–1594.
- Svartz N. The treatment of rheumatic polyarthritis with acid AZO compounds. Rheumatism. 1948 Apr;4(1):180–185.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017 Jul 27;377(4):317–328.
- Jayne DRW, Merkel PA, Schall TJ, et al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021 Feb 18;384(7):599–¬609.
- Walsh M, Merkel PA, Peh CA, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020 Feb 13;382(7):622–631.
Autoantibodies Before SLE
Preclinical autoimmunity
BY GUY KATZ, MD, JACQUELYN NESTOR, MD, PHD, STEVE WITTE, MD, PHD, ZANDRA WALTON, MD, PHD, GREG CHALLENER, MD, & MARCY BOLSTER, MD
Base Article
Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003 Oct 16;349(16):1526–1533.1
Team Overview
Virtually every player with systemic lupus erythematosus (SLE) harbors autoantibodies as part of their offensive strategies. For decades, these autoantibodies have been powerhouses in the workup and long-term management for patients with SLE and many other systemic autoimmune diseases. Indeed, the ability to analyze the meaning of these tests in different clinical contexts is one of the defining skills of a rheumatologist. However, long after they were discovered to be associated with SLE, critical questions surrounding these autoantibodies remained. What is their role in disease pathogenesis? When in the disease process do they develop? How should we counsel patients with positive autoantibodies but no clinical evidence of associated autoimmune disease? In the landmark article by Arbuckle et al., answers to these questions were finally uncovered, providing novel insights into SLE and autoimmunity.
Recruiting players from a powerful database of 30 million prospectively collected serum samples, patients with SLE (n=130) and four matched controls each were identified.1 These samples were then interrogated for the presence of anti-nuclear antibodies (ANA) at a titer of 1:120 titer or greater, as well as antibodies to Ro (SSA), La (SSB), dsDNA, Smith (Sm), RNP and phospholipids.
This team effort revealed that SLE-associated antibodies attracted the attention of scouts long before clinical disease manifestations and their tournament debut. Ro and La appeared starting the shot clock, on average, over three years before diagnosis. Likewise, development of other antibodies also predated SLE debuts, and seroconversion cheered the players toward diagnosis, with ANA and antiphospholipid antibodies appearing three years, dsDNA two years, Sm 1.5 years, and RNP nearly 11 months prior to diagnosis. Autoantibodies predicted future performance, with the vast majority of SLE players (about 90%) harboring positive antibodies for more than two years prior to the onset of symptoms. And these estimates on the lead time of antibody positivity prior to clinical disease onset and diagnosis are likely underestimated because many of the earliest available samples already exhibited autoantibodies.
Impact on Rheumatology
The Arbuckle team not only filled important knowledge gaps in SLE pathophysiology, but it also changed the game for research on preclinical autoimmunity in numerous diseases, which is now one of the areas of investigation in rheumatology at the top of the standings. This team provided answers to real-world clinical questions that rheumatologists and patients encounter every day. Autoantibody positivity—particularly ANA—is one of the most common reasons for rheumatology referral. Only a minority of patients with positive ANA develop ANA-related systemic autoimmune disease, yet it is crucial to identify and provide a defense for those who will develop diseases such as SLE.
Nearly two decades after its league debut, we continue to refer to this championship winning team for evidence-based plays on how to coach patients with positive autoantibodies in the absence of clinical manifestations of autoimmune disease. Using the offensive style demonstrated in this study, clinicians can confidently rank patients with positive autoantibodies and coach them on their odds of developing autoimmune disease using specific game times.
This paper is a much-needed playbook detailing immune-related events prior to SLE tip-off, which was previously uncharted territory. It provided key plays—answering some of the most common clinical questions rheumatologists face—that led to a momentum shift in our understanding of SLE pathogenesis, and it established the field of preclinical autoimmunity. The study directly impacts patients and clinicians every day, and its winning results have gone on to shape our understanding of the playbook of not just SLE, but of many autoantibody-associated autoimmune diseases.
Chances in the Tournament
Since its tournament debut in 2003, this manuscript has consistently been a dynasty in the field of rheumatology, and it is certain to be a fan favorite in RheumMadness 2023. Due to its clinical applicability and broad research implications that extend far beyond SLE, this landmark article has a strong chance of cutting down the net as the tournament champion. Its applicability to numerous autoimmune diseases will likely lead to its landslide victory in the Ab Workout Region, where the other teams apply more narrowly to individual diseases (anti-CCP and RA, anti-neutrophilic cytoplasmic antibody [ANCA] and ANCA-associated vasculitis).
If prior tournaments are any indication, landmark clinical trials from TNF Takedown, RA Revamp, Get in the Game, and Jump Ball are likely to be layups in the tournament. However, the broad impact of this study—addressing more than a single disease—is the reason it is likely to be a slam dunk over these trials, none of which have been cited as many times (i.e., have as many wins) as the paper by Arbuckle et al.
All authors are part of the Rheumatology Fellowship Program at Massachusetts General Hospital, Boston.
Reference
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003 Oct 16;349(16):1526–1533.
Disclosures
Marcy B. Bolster, MD, has received grants from the Rheumatology Research Foundation and is a member of the ACR Board of Directors.
Team PR3
Rituximab supplants cyclophosphamide in AAV treatment
BY KHIEM VU, MD, RACHEL WOLFE, MD, & JON GOLENBIEWSKI, DO
Base Article
Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232.1
Team Overview
For many years, the combination of cyclophosphamide and high-dose glucocorticoids was standard of care for remission induction in ANCA-associated vasculitis (AAV). Introduced in the early 1970s by then 32-year-old Anthony Fauci, MD, and his mentor, Sheldon Wolff, PhD, this cyclophosphamide-based regimen transformed what used to be a near-death sentence into a manageable, chronic disease.2-5 Nevertheless, cyclophosphamide is associated with significant infectious risk as well as other potential life-changing side effects, including infertility, hemorrhagic cystitis and secondary malignancies. Better treatment options were needed.
A major shift occurred after the publication of the 2010 Rituximab in ANCA-Associated Vasculitis (RAVE) trial in The New England Journal of Medicine by Stone et al.1 A multi-center, randomized, double-blind, double-dummy, non-inferiority trial, it compared rituximab with glucocorticoids to oral cyclophosphamide with glucocorticoids for remission induction in 197 patients with newly diagnosed and relapsing AAV over six months.
The results of this study have changed the standard of care ever since; the rituximab-based regimen was found to be noninferior to cyclophosphamide in achieving glucocorticoid-free, complete disease remission at six-month follow-up. Additionally, rituximab was superior to cyclophosphamide in achieving remission for relapsing cases of AAV at baseline. Moreover, there was no significant increase in adverse effects with rituximab compared with cyclophosphamide.
Impact on Rheumatology
The 2010 RAVE trial is one of the most important developments in modern rheumatology. The preference of rituximab over cyclophosphamide for severe AAV (whether new-onset or relapsing) continues to this day, as reflected in the first ever 2021 joint guideline by the ACR/Vasculitis Foundation for the management of AAV. The toppling of cyclophosphamide from its four decades of GOAT status (i.e., greatest of all time) underscores that accepting the status quo is incompatible in a field like rheumatology, and we should always strive to improve the care of our patients.
Chances in the Tournament
It’s quite clear that we are the team to beat. We are the only team in the tournament with RAVE reviews—what a very impressive feat. Siding with another team would be like choosing cyclophosphamide over rituximab; sure, it works and may get you the “W,” but why would you choose a team that will do nothing but trouble you?
Although we are confident in our chances in the tournament, we do expect to run into some tough competition from the other non-inferiority trials. There has been lots of talk about a lupus nephritis trial that also aimed to dethrone cyclophosphamide, but with a different agent, mycophenolate. However, we are only modestly impressed, at best. After all, “The enemy of my enemy is [not always] my friend.”
Should you choose to go with another team, the ref will surely blow their whistle for a travel; naysayers tread lightly because you will watch your bracket unravel.
All authors are part of the Rheumatology Fellowship Program at Wake Forest University School of Medicine, Winston-Salem, N.C.
References
- Chung SA, Langford CA, Maz M, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2021 Aug;73(8):1366–1383.
- Cohen B. The mentor who made Dr. Anthony Fauci. The Wall Street Journal. 16 Apr 2020. https://www.wsj.com/articles/the-mentor-who-made-dr-anthony-fauci-11587040520.
- Fauci AS, Wolff SM. Wegener’s granulomatosis: Studies in eighteen patients and a review of the literature. Medicine (Baltimore). 1973 Nov;52(6):535–561.
- Maugh TH II. Researchers find new therapy for vasculitis. Los Angeles Times. 2010 Jul 14. https://www.latimes.com/archives/la-xpm-2010-jul-14-la-heb-vasculitis-20100714-story.html.
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010 Jul 15;363(3):221–232.
Etanercept for RA
TNFα inhibitors
BY JESSE REISNER, DO, CATHERINE STRAHLE, DO, JASMINE THAI, MD, & CRISTINA HURLEY, MD
Base Article
Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997 Jul;337(3):141–147.1
Team Overview
This article describes a multi-center, randomized, double-blind, placebo-controlled trial studying the efficacy of the then-novel recombinant human tumor necrosis factor receptor p75-Fc fusion protein (TNFR:Fc, or etanercept) in patients with rheumatoid arthritis (RA) who had previously failed other conventional synthetic disease-modifying antirheumatic drugs.1
Little was known about the potential efficacy of TNFR:Fc in RA before the trial. Although the role of TNFα in the pathogenesis of RA was suspected and previous trials studying human monoclonal antibodies targeting TNFα had shown benefit in RA, this was the first randomized clinical trial evaluating the use of TNFR:Fc. In fact, the authors cite the 1996 safety and dose-finding study by Moreland et al., which showed reduction in disease activity in a small number of patients with refractory RA, as the inspiration for their trial.2
Patients were randomly assigned to one of four different treatment groups: TNFR:Fc twice weekly at a dose of 0.25 mg/m2 of body surface area, 2 mg/m2, 16 mg/m2 and a placebo group. The primary end point was the percentage change in swollen and tender joint count from baseline to three months.
The group receiving TNFR:Fc achieved a significantly greater reduction in the number of swollen and tender joints, when compared to the placebo group. There was a linear dose response, demonstrating that those who received higher doses of the drug had greater improvement in measures of disease activity. By contrast, the placebo group showed an initial improvement only through week 2, but no improvement thereafter. TNFR:Fc was found to be safe at all doses administered, with mild injection site reactions and mild upper respiratory infections, but no serious adverse events.
In summary, the trial demonstrates significant dose-related positive outcomes and an acceptable safety profile with the use of TNFR:Fc in patients with refractory RA.
Impact on Rheumatology
This article was crucial in triggering the biologic renaissance in rheumatology. Etanercept became the first targeted biologic therapy to be approved by the U.S. Food & Drug Administration (FDA) for RA in 1998 and, later, the first TNFα inhibitor to be approved for juvenile idiopathic arthritis and ankylosing spondylitis.
Since the approval of etanercept, it would be an understatement to say that the treatment of rheumatic diseases has been transformed. In the past two decades, multiple, alternative TNFα inhibitors have been subsequently approved for a wide range of autoimmune diseases. For patients, this renaissance has generated dramatic improvement in disease control, quality of life and decreased morbidity. The study was one of the first to demonstrate the feasibility of studying biologic medications for the treatment of rheumatic conditions, thereby catalyzing the expansion of our arsenal for the treatment of rheumatic diseases.
Chances in the Tournament
Although a drug derived from hamster ovaries may not sound intimidating, we expect etanercept to live up to its title as the first biologic approved for RA with victories in the first two rounds. Some may argue that combination therapy will triumph because two is better than one. But who wants to take multiple medications? Not us! A matchup with the TEAR trial in the third round could prove to be a close contest and etanercept will need to put on a clinic to send its opponent home. Either way, we are excited to see if this TNF could prove to be the alpha for the 2023 tournament.
All authors are part of the Rheumatology Fellowship Program at Ohio State College of Medicine, Columbus.
References
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997 Jul;337(3):141–147.
- Moreland LW, Margolies G, Heck LW Jr., et al. Recombinant soluble tumor necrosis factor receptor (p80) fusion protein: Toxicity and dose finding trial in refractory rheumatoid arthritis. J Rheumatol. 1996 Nov;23(11):1849–1855.