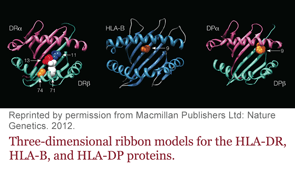
Researchers have identified five amino acids in three HLA proteins that explain most of the association between major histocompatibility complex (MHC) and seropositive rheumatoid arthritis (RA).
“About one third of the genetic risk for the disease comes from MHC—and most of that comes from three separate proteins,” says Soumya Raychaudhuri, MD, PhD, assistant professor with the divisions of rheumatology and genetics at Brigham and Women’s Hospital and Harvard Medical School in Boston. “We all understood DRB1 as a risk-conferring molecule, and now we know that HLA-B and HLA-DPB1 also play a role.”
Dr. Raychaudhuri is the lead author of the study, which was published online Jan. 29 in Nature Genetics.1 He, colleague Paul de Bakker, PhD, and others found that most of the genetic risk comes from differences in the antigen binding sites of those molecules.
“The hope is that studies such as these will help us understand which key autoantigens people are responding to,” he says. “The antigen for rheumatoid arthritis never has been easy to define.”
The strong association with MHC was first determined in the 1970s. The evidence that this association was potentially related to antigen binding was later demonstrated by the shared epitope hypotheses, first put forth in 1987 by Peter K. Gregersen, MD, Jack Silver, PhD, and Robert J. Winchester, MD.2 Despite serving as the foundation for genetic studies for RA, the shared epitope hypothesis did not fully explain the association at HLA-DRB1.
The new study builds on the shared epitope hypothesis and pins down the specific position in the molecule to which the antigen is binding, Dr. Raychaudhuri says.
“Our studies are much larger, and the genotyping and statistical analysis have advanced a lot,” he adds. “All of these things have made this study possible in a way that wouldn’t have been possible 20 years ago.”
Their results have predictive value in defining people’s relative risks for RA. However, one of the challenges of developing a genetic test for a disease is that it must be highly predictive, Dr. Raychaudhuri says.
He cites as an example that individuals who might have the greatest risk-conferring alleles at HLA-DRB1 association in both chromosomes have roughly a 15- to 20-fold risk of RA over the general population. But, since the risk of the disease in the general population is only 0.5%, such individuals would only have a 10% risk of seropositive RA.