 As practicing rheumatology providers, we are all too aware of the limited treatment options for gastrointestinal (GI) tract involvement in systemic sclerosis (SSc). The toll GI complications take on our patients’ lives is tangible at every visit, with symptoms ranging from refractory gastroesophageal reflux disease (GERD) to dependence on total parenteral nutrition. Patients and providers alike lament the paucity of data and therapies at our disposal for GI symptoms—especially when it comes to small and large bowel involvement.
As practicing rheumatology providers, we are all too aware of the limited treatment options for gastrointestinal (GI) tract involvement in systemic sclerosis (SSc). The toll GI complications take on our patients’ lives is tangible at every visit, with symptoms ranging from refractory gastroesophageal reflux disease (GERD) to dependence on total parenteral nutrition. Patients and providers alike lament the paucity of data and therapies at our disposal for GI symptoms—especially when it comes to small and large bowel involvement.
In the March 2022 issue of Arthritis Care & Research, McMahan et al. shed welcome light on this dark corner of rheumatic disease. The authors used nuclear imaging studies to determine whether abnormal GI transit contributes to GI severity and symptoms in SSc. In this article, we discuss their findings and implications for clinical practice with first author Zsuzsanna McMahan, MD, MHS, associate professor of medicine, Division of Rheumatology, Johns Hopkins University, Baltimore.1
Background
About 90% of people with SSc have GI tract involvement from their disease.2 Diet, microbiota dysbiosis and abnormalities in GI transit may contribute to GI symptomatology and severity.3 Researchers have not yet determined the clinical impact of abnormalities in GI transit in SSc patients. Because a variety of diagnostic tests and promotility agents are now available to treat GI dysmotility, understanding the connection between GI symptoms, their severity and abnormal GI transit may permit a targeted therapeutic approach for these patients.
Example: If an association between symptoms and severity and a specific area of abnormal GI transit can be demonstrated, the argument could be made for use of therapies that target specific bowel regions, such as octreotide primarily, which improves small bowel motility, and prucalopride, which is more active in the large bowel.4,5
Patients & Methods
McMahan et al. prospectively enrolled and studied 71 patients with SSc and GI symptoms from the Johns Hopkins Scleroderma Center, as well as 18 healthy controls. Symptoms of GI dysfunction included reflux, early satiety, nausea and vomiting, unintentional weight loss, distension, bloating, diarrhea and constipation.
All patients underwent whole gut transit scintigraphy, an objective nuclear medicine study used to assess GI transit from the esophagus to the colon. A radioisotope-labeled solid and liquid meal passes through the GI tract, and the extent and severity of transit abnormalities are quantified.
The investigators measured the presence of delayed transit and percent emptying in each region of the GI tract. These measurements were then compared with validated outcome measures used to assess GI severity and GI symptoms (i.e., the modified Medsger GI severity score, and University of California Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 [UCLA GIT 2.0] score, respectively).
The modified Medsger GI severity score assessed physician-reported GI clinical severity.6 This score includes five categories of GI symptomatology:
- 0=normal (no GI symptoms);
- 1=requiring GERD medications or an abnormal bowel series;
- 2=requiring high-dose GERD medications and/or having small bowel dilation on radiography;
- 3=episodes of pseudo-obstruction or malabsorption syndrome; and
- 4=severe GI dysmotility requiring either supplemental enteral nutrition or total parenteral nutrition.
The UCLA GIT 2.0 assessed patient-reported GI symptoms.7 This 34-question survey evaluates a variety of GI symptoms common in patients with SSc. Scores range from zero to 2.83, with higher scores indicating more severe symptomatology and worse health-related quality of life.
The majority of patients studied were female (85.9%) and white (78.6%), with limited cutaneous disease (70%). On cross-sectional analysis, the clinical and serologic characteristics of enrolled patients with SSc were largely comparable to other patients in the Johns Hopkins Scleroderma Center cohort, with certain exceptions. Example: Patients in this study were more commonly anticentromere antibody positive (45% vs. 27%; P<0.01), and more study patients had severe GI disease (Medsger GI severity score of 3: 14.9% vs. 5.5%; P<0.01). Researchers intentionally enriched the study with “patients who had more severe GI disease so as to learn about the impact of abnormal transit on less frequently observed, but more severe SSc GI complications.”
Analysis
Transit times and percent emptying were compared between patients with SSc and controls. As expected, patients with SSc had a significantly higher prevalence of abnormal function and delayed transit time in the esophagus, stomach and colon. Delayed small bowel transit was also more common among patients with SSc than controls, but the difference did not reach statistical significance.
Next, McMahan et al. compared the prevalence of delayed transit in each area of the gut to each category of Medsger GI severity “to determine whether distinct GI transit abnormalities [are associated] with specific clinical SSc GI complications.” In patients with a Medsger score of 4 (total parenteral nutrition dependence), 100% (i.e., three of three patients) had small bowel abnormalities on whole gut transit scintigraphy. Among patients with a Medsger score of 3 (i.e., pseudo-obstruction and malabsorption), colonic transit was severely delayed.
Lastly, McMahan et al. compared UCLA GIT 2.0 questionnaire responses with abnormalities seen on whole gut transit scintigraphy to determine whether GI symptoms were associated with specific GI transit abnormalities.
Results
In this novel study, McMahan et al. demonstrated that SSc bowel disease affects GI transit, and this abnormal transit accounts, in part, for both bowel symptoms and severity of GI disease. Patients with pseudo-obstruction or malabsorption were more likely to have severe colonic transit delays. One-third of these patients had almost zero colonic emptying whatsoever after 72 hours. Although the number of patients was small (n=3), those dependent on total parenteral nutrition were more likely to have small bowel involvement of disease. Patients with more substantial upper GI symptoms were more likely to have dysmotility of the esophagus and/or stomach.
Of note, whole gut transit scintigraphy also revealed that SSc can affect several regions of the GI tract simultaneously, reminding us that an unrevealing study in one region does not preclude abnormalities in other parts of the GI tract. The esophagus and colon were most commonly affected at the same time.
“More comprehensive testing may be indicated in symptomatic patients,” write McMahan et al.
Dr. McMahan Talks with TR
The Rheumatologist (TR): What are the take-home points of this study as they relate to clinical practice?
Dr. McMahan: First off, this study reminds us that many patients with SSc have multiple regions of the gut in which transit is negatively impacted. This [fact] is important because many of us focus mainly on the esophagus when thinking about SSc.
Second, our study demonstrates that disease duration doesn’t necessarily portend the extent of gut involvement. There were patients newly diagnosed with SSc with extensive GI issues, and there were patients with longstanding disease with only one region affected.
Third, we were particularly struck by the proportion of patients—especially those with pseudo-obstruction—who had terribly slow colonic transit. We previously thought the small bowel was the main culprit here. It’s possible that starting motility agents early in people with slow colonic transit could impact the course of progression, and we are interested in learning more about this in future studies.
TR: How could this study inform the selection of therapy for patients?
Dr. McMahan: Knowing where exactly the ‘lesions’ are in the gut is important because certain medications are helpful in some areas, but negatively impact motility in others. For example, mirtazapine can be helpful for a cachectic patient with gastroparesis who’s losing weight. But it also slows colonic transit. So for a patient who has delayed transit times in the stomach and colon, mirtazapine wouldn’t be a great choice. Knowing what you’re treating also comes into play when assessing response to therapy. Is the patient not improving due to inadequate drug dosing, or is the drug simply not active in the problem area of the gut?
TR: Could you share some pearls regarding the area of gut involvement and drug selection? What do you reach for in your own clinic?
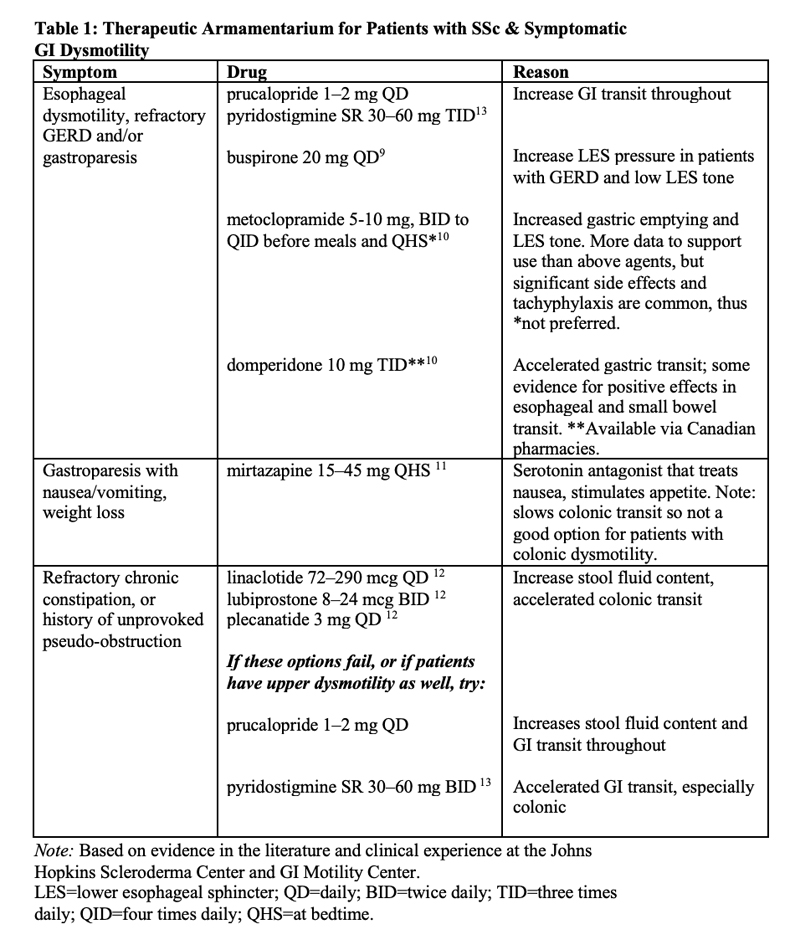
Dr. McMahan: For esophageal dysmotility, refractory GERD and/or gastroparesis, I like prucalopride or pyridostigmine. There [are] more data for metoclopramide, but its side effects can be significant, so I try to avoid it when I can.
For refractory chronic constipation and in patients with a prior history of unprovoked pseudo-obstruction, motility agents, such as linaclotide, prucalopride and plecanatide, address slow colonic transit. Pyridostigmine and domperidone—available in Canada—accelerate overall GI transit, so they could be good choices for patients with multi-level GI lesions [Table 1].
TR: Is whole gut transit scintigraphy widely available?
Dr. McMahan: Currently, it’s only available at select academic centers. My hope is that research like ours will lead to more widespread availability down the line. Of note, the SmartPill motility capsule is much more widely available and correlates pretty well with whole gut transit scintigraphy.8
TR: Do you have any tips for our readers on insurance approval, especially when it comes to more expensive prokinetic or chronic constipation therapies?
Dr. McMahan: The best advice I can give is to document every single drug failure in a note, including over-the-counter options, such as polyethylene glycol or Smooth Move tea. Studies like ours can also be referenced in letters of appeal and peer-to-peer reviews.
TR: Where would you like to see research go from here?
Dr. McMahan: For starters, I’d like to be able to better risk-stratify patients. For a high-risk progressor, perhaps early therapy could prevent progression. For a person at low risk, we could take the burden of worry away and eliminate unneeded GI referrals and tests.
Next, I’d like to better understand the actual biological mechanisms that are driving GI disease in SSc. That way, we can develop treatments that don’t just target symptoms, but prevent or stop disease.
And finally, I’d like to see clinical trials look at combination therapies that target both symptoms and physiologic dysfunction. For example, would combining a promotility agent with antacid therapy at baseline in high-risk subgroups be more helpful than focusing on amplifying antacid doses alone?
Conclusion
For years, we’ve had limited treatment options for GI disease in our SSc patients. McMahan et al. have taken pivotal steps to changing that paradigm. With better knowledge about the exact location of GI involvement, we can hope for personalized therapies for symptom relief or, better yet—prevention and cure—in the future.
Samantha C. Shapiro, MD, is an academic rheumatologist and an affiliate faculty member of the Dell Medical School at the University of Texas at Austin. She received her training in internal medicine and rheumatology at Johns Hopkins University, Baltimore. She is also a member of the ACR Insurance Subcommittee.
References
- McMahan ZH, Tucker AE, Perin J, et al. Relationship between gastrointestinal transit, Medsger Gastrointestinal Severity, and University of California–Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 symptoms in patients with systemic sclerosis. Arthritis Care Res (Hoboken). 2022 Mar;74(3):442–450.
- Sjogren RW. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum. 1994 Sep;37(9):1265–1282
- Shreiner AB, Murray C, Denton C, et al. Gastrointestinal manifestations of systemic sclerosis. J Scleroderma Relat Disord. 2016;1(3):247–256.
- Vigone B, Caronni M, Severino A, et al. Preliminary safety and efficacy profile of prucalopride in the treatment of systemic sclerosis (SSc)-related intestinal involvement: Results from the open label cross-over PROGASS study. Arthritis Res Ther. 2017 Jun 20;19(1):145.
- Soudah HC, Hasler WL, Owyang C. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N Engl J Med. 1991;325(21):1461–1467.
- Medsger TA, Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: Development and testing. J Rheumatol. 1999 Oct;26(10):2159–2167.
- Khanna D, Hays RD, Maranian P, et al. Reliability and validity of the University of California, Los Angeles scleroderma clinical trial consortium gastrointestinal tract instrument. Arthritis Rheum. 2009 Sep 15;61(9):1257–1263.
- Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: Comparison of the smartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci. 2009 Oct;54(10):2167–2174.
- Karamanolis GP, Panopoulos S, Denaxas K et al. The 5-HT1A receptor agonist buspirone improves esophageal motor function and symptoms in systemic sclerosis: A 4-week, open-label trial. Arthritis Res Ther. 2016 Sep 1;18(1):195.
- Foocharoen C, Chunlertrith K, Mairiang P, et al. Effectiveness of add-on therapy with domperidone vs alginic acid in proton pump inhibitor partial response gastro-oesophageal reflux disease in systemic sclerosis: Randomized placebo-controlled trial. Rheumatology (Oxford). 2017 Feb;56(2):214–222.
- Malamood M, Roberts A, Kataria R, et al. Mirtazapine for symptom control in refractory gastroparesis. Drug Des Devel Ther. 2017 Mar 30;11:1035–1041.
- Dein E, Kuo PL, Hong YS, et al. Evaluation of risk factors for pseudo-obstruction in systemic sclerosis. Semin Arthritis Rheum. 2019 Dec;49(3):405–410.
- Ahuja NK, Mische L, Clarke JO, et al. Pyridostigmine for the treatment of gastrointestinal symptoms in systemic sclerosis. Semin Arthritis Rheum. 2018 Aug;48(1):111–116.