The study included patients who were biologic naive and those who had an inadequate response to tumor necrosis factor inhibitors. Patients in the latter group made up 31.5% of those who received 300 mg of secukinumab, 30.2% of those who received 150 mg of secukinumab, 27.2% of those who received 150 mg of secukinumab with no loading dose at baseline, and 30% of those who received placebo.
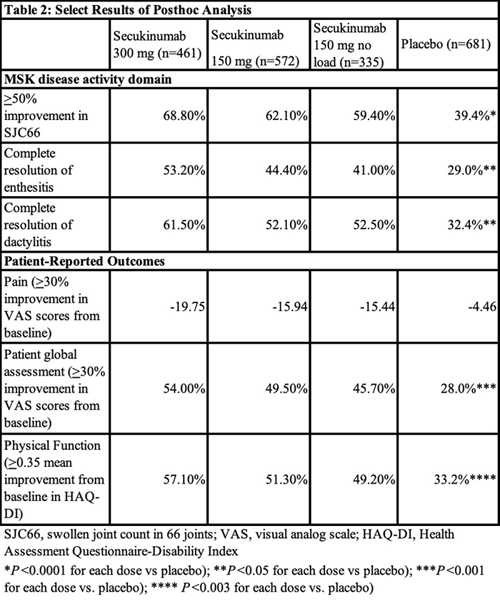
The investigators assessed the efficacy of secukinumab at week 16 in all domains of the updated GRAPPA-OMERACT PsA core domain set. They found secukinumab demonstrated significant efficacy across all PsA core domains. See Table 2 (below) for a sampling of the results.
The greatest efficacy in all core domains was seen in patients treated with the 300 mg dose of secukinumab. Patients with skin disease activity receiving 300 mg of secukinumab had the best response rates across skin measurements, with 33.2% achieving complete resolution of psoriasis by week 16 and 52.3% achieving clear or almost clear skin.
Dr. Orbai emphasizes these findings provide much needed information about the efficacy of secukinumab for PsA and translate into helpful guidance for rheumatologists when talking to patients.
“We already knew from the randomized trials that secukinumab is efficacious for PsA, but now we can … predict which patients, for example, [may] have resolution of skin disease or enthesitis,” she says. “This is practical information for rheumatologists.”
Although the study doesn’t provide any novel data, Laura C. Coates, PhD, clinician scientist and senior clinical research fellow, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, Botnar Research Centre, Oxford, U.K., and first author of the 2015 GRAPPA treatment recommendations for psoriasis and PsA, underscores that the Orbai study provides “a very concise summary, bringing together evidence of efficacy across all of the disease activity domains and key areas of patient impact.” 2
“I think it is important that we consider all domains of PsA, in terms of assessment and choosing therapies, and I think the paper brings all of that data together for secukinumab,” she says.
According to Dr. Orbai, more research is still needed to find patients who are complete responders for whom PsA may have a different mechanism of action and for whom first-line therapy with secukinumab may make sense.
Mary Beth Nierengarten is a freelance medical journalist based in Minneapolis.
References
- Orbai AM, McInnes IB, Coates LC, et al. Effect of secukinumab on the different GRAPPA-OMERACT core domains in psoriatic arthritis: A pooled analysis of 2,049 patients. J Rheumatol. 2020 Jun 1;47(6):854–864. Epub 2019 Oct 15.
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016 May;68(5):1060–1071. Epub 2016 Mar 23.