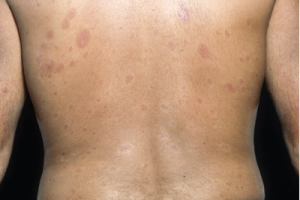
Plaques of thickened skin on the back of a patient with diffuse systemic sclerosis.
SPL / Science Source
A recent paper in Arthritis & Rheumatology opens up the possibility of a new research avenue to treat systemic sclerosis: dipeptidyl peptidase 4 (DPP4) inhibitors, a previously approved therapy for type 2 diabetes.1 Work in mouse models and on skin samples from systemic sclerosis patients suggests these drugs pose a promising area of future translational research.
TGF-β, DPP4 & Fibrosis in Systemic Sclerosis
Fibroblasts play a critical role in wound healing. When activated by local environmental conditions, the cells differentiate into myofibroblasts. These activated cells release profibrotic mediators, contract the tissue and provide the central source of extracellular matrix needed for wound repair.2
Alina Soare, MD, a rheumatologist in the Department of Medicine 3, Rheumatology and Immunology, University Hospital Erlangen, Germany, is first author of the recent paper on dipeptidyl peptidase 4 inhibitors. Dr. Soare points out that in normal wound healing, fibroblast activation stops as soon as the damage has been repaired. However, she notes, “in fibrotic diseases, tissue remodeling continues, with persistence of myofibroblasts in affected tissues.” For reasons that are not fully understood, fibroblasts sometimes maintain this activated state in an uncontrolled manner, causing excessive release of extracellular matrix and disrupting physiological tissue architecture.1,2
This fibrotic tissue remodeling plays a pathophysiological role in many diseases, including atherosclerosis, liver cirrhosis, renal fibrosis and many cancers.2 It is also one of the key characteristics of systemic sclerosis, where chronic activation of fibroblasts leads to the severe disability and morbidity associated with the condition.1
Broad evidence from different fibrotic diseases has demonstrated that persistent activation of transforming growth factor β (TGF-β) plays a key role in this process of fibrosis, promoting the activation of fibroblasts and their differentiation into their fibrotic myofibroblast state.1,3 Dr. Soare notes, “TGF-β plays a crucial role in systemic sclerosis-related fibrosis, as it stimulates the synthesis of extracellular matrix protein, decreases the release of collagen-degrading metalloproteinase and stimulates the production of protease inhibitors, which prevent the breakdown of extracellular matrix.”
The protein dipeptidyl peptidase 4 is a serine protease that can modulate intracellular signaling. Among other properties, it is thought to play a role in immune regulation.1 Previous work has demonstrated that fibroblasts that express DPP4 constitute a distinct fibroblast subset, a group that seems to play a critical role in fibrosis and wound repair.4 This and other factors led the team to explore the role of DPP4 in fibrosis in the context of systemic sclerosis.
Study Findings
Using multiple lines of evidence, the researchers assessed the role of DPP4 expression on fibrosis in systemic sclerosis. They used skin biopsy samples to isolate dermal fibroblasts from 23 people with systemic sclerosis, matched with 21 healthy controls. In vivo studies included three classic mouse models used to mimic the symptomatology of systemic sclerosis. These were bleomycin-induced skin and lung fibrosis, sclerodermatous chronic graft-versus-host disease, and the tight skin mouse model (fibrillin-1 mutation mouse).1,3
To help clarify the role of DPP4, the team employed a strain of mice genetically bred to be unable to express DPP4—DPP4 knockouts—as well as a control strain (wild type). This helped the team tease apart the role of DPP4, as they saw how the different mice responded to induction of the systemic sclerosis model. They also explored sitagliptin, a DPP4 inhibitor, to see how its usage affected induced systemic sclerosis in these various mouse models.1
Dr. Soare and colleagues found the expression of DPP4 was increased in patients with systemic sclerosis compared to controls, with fibroblasts as the dominant cell type expressing DPP4. For example, almost 76% of the fibroblasts of systemic sclerosis patients expressed DPP4, compared to about 29% of fibroblasts in people with healthy skin. This pattern also held in mouse models of the disease. The population of fibroblasts expressing DPP4 seemed to mark a subset of highly activated fibroblasts, which expressed higher levels of collagen and of genetic markers for myofibroblasts. However, serum levels of DPP4 did not differ, consistent with earlier work, suggesting DPP4 is locally regulated.1
Moreover, this expression of DPP4 appeared to increase in proportion to TGF‑β. Overexpression of DPP4 promoted activation of fibroblasts. Conversely, Dr. Soare notes, “Inactivation of DPP4 blocked TGF-β-induced fibroblast-to-myofibroblast differentiation and reduced the release of collagen in vitro.”
She adds, “Genetic or pharmacologic inhibition of DPP4 also ameliorated experimental dermal and pulmonary fibrosis induced by bleomycin or by sclerodermatous processes [chronic graft-vs.-host disease].” Significantly, not only did pharmacological inhibition of DPP4 prevent further fibrosis progression, it also induced regression of existing fibrosis to below pre-treatment levels.1
Moreover, in addition to the direct effect on fibroblasts, inhibition of DPP4 seemed to also reduce inflammation.1 Dr. Soare explains, “In murine models of systemic sclerosis, treatment with DPP4 inhibitors reduced leukocyte counts and in particular T cell and B cell infiltration, both of which are centrally involved in the pathogenesis of the disease.”
Through molecular analysis, the team demonstrated the importance of the intracellular signaling mediator ERK (extracellular signal-regulated kinase), whose inhibition has previously been shown to ameliorate fibrosis in experimental models.5 Dr. Soare remarks, “Inhibition of the non-canonical TGF-β signaling mediator, ERK, inhibits the stimulatory effects of TGF-β on DPP4 expression.” Moreover, treatment of incubated human fibroblasts with DPP4 inhibitors prevented TGF-β from having its normal stimulatory effects on ERK signaling. Dr. Soare reports that other known intracellular cascades regulated by TGF-β were not found to be affected by inhibition of DPP4.1
Further Investigations of DPP4 Inhibitors
Dr. Soare notes that the potent antifibrotic effects of DPP4 inhibitors may have direct translational implications. DPP4 inhibitors have already been shown to have a good tolerability and safety profile, making them an attractive target for clinical investigation. In 2006, the U.S. Food and Drug Administration approved the use of oral DPP4 inhibitors to treat type 2 diabetes. DPP4 inhibitors increase the levels of active insulinotropic polypeptide and glucagon-like peptide-1, resulting in stimulation of insulin and inhibition of glucagon.6 Data from other clinical studies has shown promising results for DPP4 inhibitors in other disease states, such as renal disease.7
“To prove the effect in patients with systemic sclerosis, it would definitely be interesting to test DPP4 inhibitors in a clinical trial,” says Dr. Soare. “Using the data from the post-marketing studies available in patients with type 2 diabetes treated with DPP4 inhibitors would speed up the process of approval of these agents for another indication.”
DPP4 inhibitors are not the only components of the TGF-β pathway that researchers have pursued. In 2015, researchers reported promising results of a phase 2 trial of systemic sclerosis patients with fresolimumab, an inhibitor of all three isoforms of TGF-β.8 Dr. Soare points out that in contrast to fresolimumab, inhibiting DPP4 expression would not affect all downstream signaling pathways of TGF-β. This might make it more suitable for use in light of its side effect profile.
Clinical trials are needed before any strong conclusions can be drawn. However, DPP4 inhibitors may represent a new avenue of hope for this devastating disease.
Ruth Jessen Hickman, MD, is a graduate of the Indiana University School of Medicine. She is a freelance medical and science writer living in Bloomington, Ind.
References
- Soare A, Györfi HA, Matei AE, et al. Dipeptidylpeptidase 4 as a marker of activated fibroblasts and a potential target for the treatment of fibrosis in systemic sclerosis. Arthritis Rheumatol. 2020 Jan;72(1):137–149.
- Zent J, Guo LW. Signaling mechanisms of myofibroblastic activation: Outside-in and inside-out. Cell Physiol Biochem. 2018;49(3):848–868.
- Ayers NB, Sun CM, Chen SY. Transforming growth factor-β signaling in systemic sclerosis. J Biomed Res. 2018 Jan 26;32(1):3–12.
- Rinkevich Y, Walmsley GG, Hu MS, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015 Apr 17;348(6232):aaa2151.
- Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: Translation of basic research to human disease. Biochim Biophys Acta. 2013 Jul;1832(7):897–904.
- Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: Preclinical biology and mechanisms of action. Diabetes Care. 2007 Jun;30(6):1335–43.
- Kanasaki K. The role of renal dipeptidyl peptidase-4 in kidney disease: Renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin Sci (Lond). 2018 Feb 28;132(4):489–507.
- Rice LM, Padilla CM, McLaughlin SR, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015 Jul 1;125(7):2795–2807.