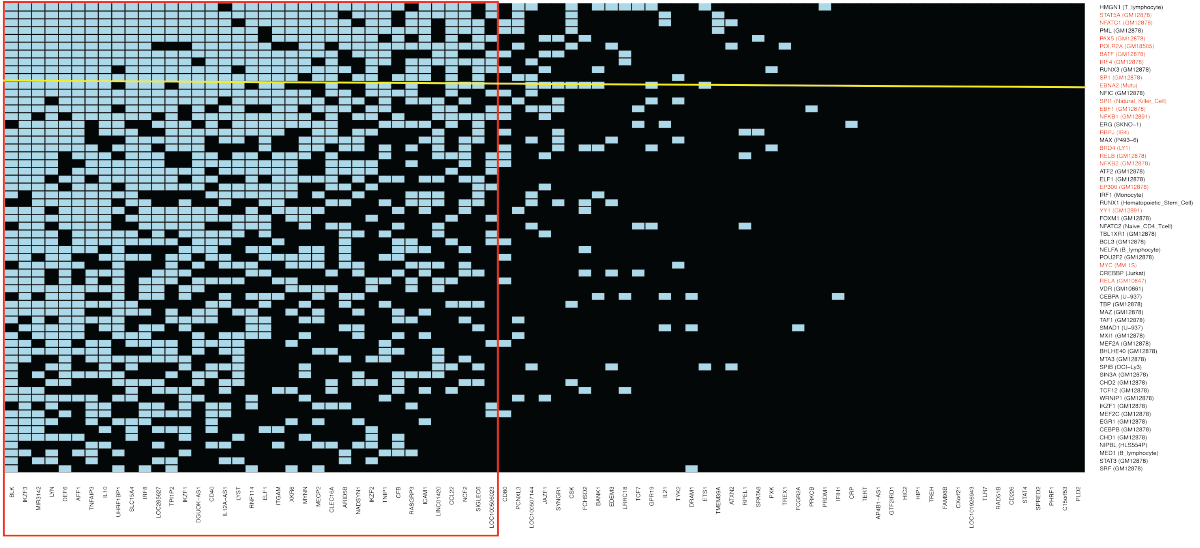
(click for larger image) Figure 1: Systemic Lupus Erythematosus
The blue boxes indicate the intersections found when transcription factors and co-factors (TFs) form complexes with variants located in 83 risk loci for systemic lupus erythematosus (SLE). The 83 SLE loci are from Europeans, African Americans, Asians and Hispanics. Each transcription factor is identified by its gene name; the cell type from which the DNA binding data were obtained are given in parentheses. Every TF shown has a probability of Pc<10-6 of the intersections with the SLE loci shown having occurred by chance after Bonferroni correction. The TFs in red are known to form super-enhancers in Epstein-Barr virus (EBV) transformed B cells. The EBV gene product EBNA2 is identified by a yellow line. All of the other TFs shown are encoded by the human genome. The red rectangle encloses a cluster of SLE loci and TFs that possibly function together to change the risk of SLE by gene expression regulatory mechanisms in B lymphocytes transformed by EBV. Note: Data are abstracted from Nature Genetics and adapted with permission by Sreeja Parameswaran.1
Clinical Implication?
This study points to the potential to develop future therapies that can manipulate the action of the proteins in persons harboring risk alleles at EBNA2-bound loci, conclude the study investigators.
Based on the specific findings of the study, the investigators plan to further investigate particular transcription factors and cell types for 94 phenotypes they’ve nominated to help provide a better understanding of the molecular and cellular origins of disease risk. “As new genetic association and TF [transcription factor] binding data are collected, approaches such as this will undoubtedly identify further disease mechanisms,” concludes Dr. Harley for the investigators.
The investigators are hoping experts on various diseases will share their results and collaborate to better understand these mechanisms. To that end, they have made their computer tools available online, along with their study data and results.
“My hope is that the study spurs further research into this connection,” says Dr. Nigrovic, “for example, to test experimentally the role of ENBA2 in the regulation of RA and lupus target genes.”
Most important to Dr. Nigrovic is what the study findings suggest may be the best target of all—prevention of autoimmunity. “To me, the most exciting implication is for prevention,” he says. “EBV infection increases with age, and ultimately, most people become infected. If we could prevent infection—by vaccination, for example—then it is possible we could reduce the incidence of autoimmune disease substantially. That’s an amazing prospect.”