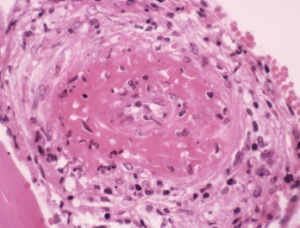
Light micrograph of a section through a human artery, showing a large thrombus (blood clot, center) due to cryoglobulinemia.
CNRI / Science Source
A study that focused on the detection and immunological characteristics of cryoglobulins provides insights for rheumatologists and other rheumatology providers, as well as lab professionals.
Co-researchers Marie N. Kolopp-Sarda, PharmD, PhD, and Pierre Miossec, MD, PhD, Clinical Immunology Unit, Department of Immunology and Rheumatology, University of Lyon, France, included in their retrospective study, published in Arthritis & Rheumatology, a large cohort of 13,439 patients who were tested for cryoglobulins—immunoglobulins (Ig) that precipitate in cold temperatures—from January 2010 to December 2016 in Lyon.1
Previous studies on the characteristics of cryoglobulins vary due to population selection bias and because of different methods to detect and characterize cryoglobulins, the researchers noted. “The topic is usually seen as complex and even mysterious,” Dr. Miossec says.
The Study
The researchers analyzed cryoglobulin isotype, clonality, concentration and rheumatoid factor in cryoprecipitate, as well as serum complement and rheumatoid factor. They also analyzed markers of gammopathy, viral infection and autoimmunity.
If no cryoprecipitates were detected, one or two blood samples were analyzed to confirm the negative result. Cryoglobulins were identified by electrophoresis-immunofixation of the dissolved cryoprecipitate with various antisera and were then classified by their immunoglobulin clonality profile, according to the classification of cryoglobulin (type I: monoclonal Ig; type II: monoclonal Ig with rheumatoid factor activity; type III: polyclonal Ig with rheumatoid factor activity). Rheumatoid factor activity was measured in the cryoprecipitate and in the serum aliquot. Complement functional activity and C3 and C4 concentration also were measured. Researchers also combined biological data with the most frequent underlying diseases associated with cryoglobulin detection.
Autoantibodies identified as markers of an autoimmune context included double-stranded DNA antibodies, often associated with systemic lupus erythematosus; anti-SSA/Ro60 Ab, often associated with Sjögren’s syndrome; and anti-cyclic citrullinated peptide Ab, associated with rheumatoid arthritis.
Results from the Cryoglobulin Analysis
Cryoglobulins were detected in 1,675 (1,018 women and 657 men; with a mean age of 54 years) of the 13,439 patients. In the full cohort, 89% tested negative for the first sample; 18.5% were retested to confirm the result, and 8.9% then tested positive.
The specialties of origin for the patients were internal medicine (42.9%), neurology (15.1%), hepatology (9.6%), nephrology (9.5%), rheumatology (7.8%), dermatology (5.5%), pulmonary medicine/cardiology (4.2%), hematology (3.7%) and infectious diseases (1.8%).
The study also found that complement activation by immune complexes—specifically, IgM cyroglobulin associated with IgG—is one of the mechanisms contributing to cryoglobulinemic vasculitis, especially hepatitis C-associated cryoglobulinemic vasculitis.
Type I cryoglobulins were present in 9.3% of those who tested positive. About 79% of the type I cryoglobulins were composed of monoclonal IgM, and nearly 21% were monoclonal IgG.
Type II cryoglobulins were found in 788 patients (47%), with 64.9% of the cryoglobulins being monoclonal IgMκ, 19.3% IgMλ, 10.4% IgGκ, 4.4% IgGλ and 1% IgAκ or IgAλ.
Type III cryoglobulins were found in 43.7%. These were most frequently polyclonal IgG and IgM, followed by polyclonal IgG or IgM alone.
Both cryoprecipitate and serum were rheumatoid factor positive in 21.6% of patients with type II cryoglobulins and 10.1% of patients with type III cryoglobulins.
Among the larger patient cohort of more than 13,000 patients, 40% were tested for their autoimmune antibody status. Of those, 11.2% were positive for at least one autoantibody. Among them, 25.4% were positive for cryoglobulin. Type II cryoglobulin was significantly less frequent than type III cryoglobulin in patients with an autoimmune diagnosis. Improvement of techniques used to detect rheumatoid factor in a cryoprecipitate is needed, the researchers note in the study.
The study also found complement activation by immune complexes—specifically, IgM cyroglobulin associated with IgG—is one of the mechanisms contributing to cryoglobulinemic vasculitis, especially hepatitis C-associated cryoglobulinemic vasculitis, the researchers write.