Background/Purpose
Polymyalgia rheumatica (PMR) treatment is primarily based on long-term corticosteroids, which results in significant toxicities. Studies have shown that patients with PMR are exposed to years of corticosteroid treatment.1,2 In a single academic center cohort, we found that 76% of patients remained on steroids at the end of two years.3
In a second cohort of PMR patients, we explored baseline characteristics and established the success rate of discontinuing steroid treatment at years 1 and 2 after initiating the treatment.
Methods
A retrospective, observational study was conducted using data collected from a rheumatology clinic at an academic medical center. We included patients with a primary diagnosis of PMR or PMR associated with giant cell arteritis (GCA) and a minimum of one year of follow-up. Patients with other rheumatic disorders were excluded. Of 403 PMR charts reviewed, 346 patients seen from 2011 to 2021 met the inclusion criteria.
Results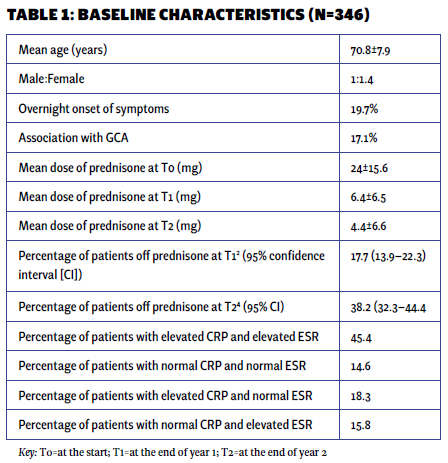
The mean age of the patient population was 71 years, with 59% being women and 41% men. Approximately 20% of the patients experienced an abrupt onset of symptoms. Among the PMR patients, 17.1% had a concurrent diagnosis of GCA, which usually developed alongside or after the PMR diagnosis. Only 2% of patients presented with remitting seronegative symmetrical synovitis with pitting edema (RS3PE).
At the beginning of treatment (T0), the mean prednisone dose was 24 mg daily. At one year (T1), it decreased to 6.4 mg, and at two years (T2), it further reduced to 4.3 mg daily. At the end of one year, 17.7% of patients had discontinued prednisone, while only 38% had achieved discontinuation by the end of two years.
The majority of patients exhibited elevated markers of inflammation (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]), with only 14.6% presenting with normal values initially. The only patient characteristic associated with successful discontinuation was the initial steroid dose. Multivariable logistic regression analysis showed that with each unit increase in the initial prednisone dose, the odds of successfully discontinuing prednisone at 12 months decreased by 3% (P=0.034). However, the significance of this association was not sustained at the 24-month mark. No other baseline characteristics were associated with successful prednisone discontinuation (see Tables 1 and 2).
Conclusion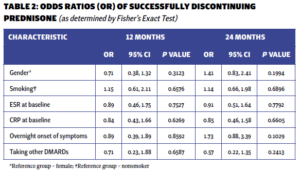
The clinical course of PMR is characterized by prolonged corticosteroid use, with a discouraging rate of successful prednisone discontinuation. Our study validates previous observations and provides additional insights into the clinical characteristics of PMR patients.3 Similar to prior research, our study demonstrates that the initial corticosteroid dosage predicts the likelihood of successful discontinuation.4,5 However, the significance of this association diminishes at the 24-month mark, highlighting the need for further investigation. We acknowledge the limitations of our retrospective study, including potential selection bias due to the inclusion of patients with only one year of follow-up. Nonetheless, these findings underscore the urgency for additional research to identify and incorporate alternative disease-modifying anti-rheumatic drugs into the PMR treatment regimen.


