Reviewing the research presented at ACR Convergence 2024
WASHINGTON, D.C.—Despite significant advances that have taken place over the past several years, systemic lupus erythematosus (SLE) still presents great challenges to rheumatologists, given its unpredictable clinical course and the eventual consequences the disease itself and the complexity of its treatments impose on individual patients, their families and society as a whole. This year’s ACR annual meeting, ACR Convergence 2024, brought us numerous reports on several important issues related to SLE. The abstracts selected for these comments include a variety of topics, from those about how to identify anti-nuclear antibody (ANA) positive patients that may evolve toward SLE to the promising use of most effective therapies and a lot more in between, including biomarkers for lupus nephritis (LN), adherence to treatment guidelines, etc.
Progression to SLE
Abstracts 1526: Markus H et al. & L11: Choi M et al.1,2
Rheumatologists are often called to evaluate patients with some manifestations suggestive of SLE but who have yet to have enough clinical and laboratory manifestations to allow such a diagnosis to be made. Thus, the possibility of distinguishing between patients who will evolve into SLE and those who will not is of great clinical significance. Two abstracts addressed this subject.1,2
In the first one, investigators used electronic health records from the TrinNetX Research Network to determine the clinical and laboratory features that may be indicative of a patient advancing from “incomplete” to full SLE. To be included in the study, subjects had to have clinical data two years prior to ANA testing with the diagnosis being explored three months and five years after they tested positive for ANA. The information pieces abstracted (diagnosis, procedures, medications and laboratory/vital records) were used to train machine learning models. Twenty predictors for progression to SLE were identified; subjects with OR predictors <1 were unlikely to evolve into SLE, whereas those with ORs >1 were likely to progress toward the full diagnosis. Figure 1 (below) shows the prevalence of progressing from ANA+ to SLE: as the score increases, the likelihood of progressing to SLE also increases.
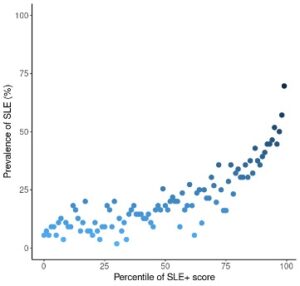
Figure 1: Prevalence of Progressing from ANA+ to SLE in 100 Percentile Groups of GBM-Predicted Scores (SLE+ score) in Testing Dataset
In the second abstract, investigators compared the assessments of autoantibody testing (images) as performed by an experienced laboratory professional and by artificial intelligence (AI). The data presented support the accuracy of AI in interpreting these images in two sets of samples; the first one was used to develop the model and included over 3,000 ANA images; a second one (n=50) was used to validate the model. The aim was to distinguish patterns known to be associated with a low probability of patients evolving into a systemic autoimmune rheumatic disease (SARD), AC-2 and those with a high probability (AC-4 and AC-20) of doing so. In all, AI performed very well reporting very significant areas under the curve (AUC).
The data presented in these two abstracts are potentially quite useful for the identification of subjects at risk for developing SLE. Before they are widely applied in the clinical setting, however, these models may need further validation. Recognizing SLE early may allow the initiation of an adequate treatment to prevent disease progression, damage accrual and early mortality.
The Influence of Trauma on Features of Type 2 SLE
Abstract 1520: Rogers et al.3
Patients with SLE presenting predominantly with such symptoms as fatigue, widespread pain, sleep disturbances and cognitive dysfunction have been named type 2 SLE, to differentiate them from patients experiencing major organ system involvement or type 1 SLE.4,5 Such distinction has obvious therapeutic clinical implications because commonly used modalities for SLE may have limited efficacy in type 2 patients.
Traumatic events have been associated with these kinds of symptoms; thus, these investigators sought to determine the presence of this relationship in adult patients with lupus from a U.S. academic center. They inquired about polysymptomatic distress and anxiety using the Brief Trauma Questionnaire (BTQ);6 questions about emotional abuse, illness in family members and pregnancy-associated issues were added to the BTQ. These investigators grouped physical, sexual and emotional abuse experiences under the term abusive trauma.
The study included 262 SLE patients, 85% of whom reported some trauma. Overall, women were five times more likely than men to experience abusive trauma. Patients experiencing abusive trauma had a higher mean number of type 2 symptoms and required medications for their treatment. The authors concluded that patients with SLE experiencing trauma, particularly of the abusive type, were likely to experience type 2 symptoms with higher frequency than those not exposed to it.
These data are quite relevant to the practice of our subspecialty, considering that eliciting such history may allow the healthcare team to approach patients in a more holistic way and manage these symptoms using all available resources rather than assume they require additional immunosuppressive therapy. Thus, instruments like this for the assessment of SLE patients’ traumatic experiences may be of significant help in choosing the most appropriate treatment for them. Although the questions added to this questionnaire have not yet been validated, the authors are willing to share the questionnaire so others can use it.
Lupus Nephritis: Disease Activity & Treatment Response
Abstract 1642: Fava et al. & Abstract 1685: Aundhia et al.7,8
Lupus nephritis (LN) is one of the most serious manifestations of SLE and, even if treated, may lead to end-stage renal disease and the need for renal dialysis or transplantation. Thus, recognizing whether patients are responding adequately to treatment by non-invasive methods is a welcome addition to the armamentarium of the practicing rheumatologist.
In the first study, U.S. investigators examined a panel of 1,200 proteins in urine samples of patients with LN to find out which ones could predict a National Institutes of Health (NIH) Activity Index >2.7 Twelve proteins were selected using 80% of the patients’ data, which were then validated with the remaining 20% of the data. Complete (CRR) or partial renal responses (PRR) at one-year post-biopsy were examined. The panel selected outperformed (90%) other commonly used LN markers, such as C3 (73%), C4 (67%), anti-dsDNA (60%), and urinary protein creatinine ratio (UPCR [59%]) as noted in Figure 2B (below). In the validation set, the area under the curve (AUC) was 86%, still higher than the percentages for the traditional markers as noted in Figure 2A (below).
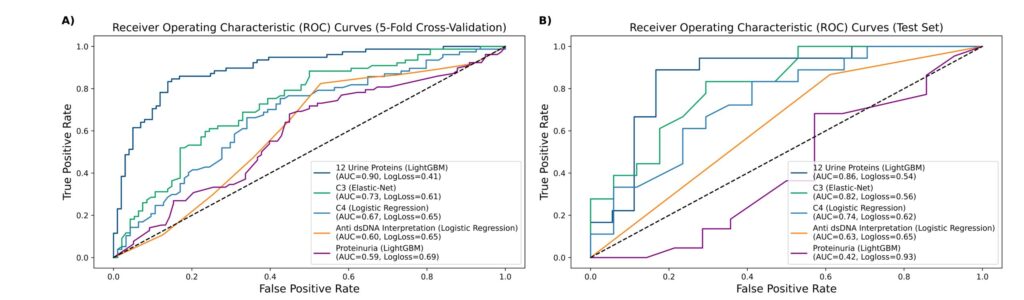
Figures 2A & 2B: Performance Characteristics of 12 Urinary Biomarkers & Conventional Biomarkers in Predicting an NIH Activity Index >2 at Baseline
The three best performing proteins in this study were interferon gamma receptor 1, CD163 and carcinoembryonic antigen-related cell adhesion molecules-1 (CEACAM-1).
In the second abstract, the previously identified renal activity index for lupus (RAIL) which has been shown to predict renal response in LN patients was studied in the context of a randomized clinical trial of mycophenolate mofetil (MMF) and steroids ± abatacept.8 This index includes six urinary biomarkers: neutrophil gelatinase-associated lipocain (NGAL), kidney injury molecule-1 (KIM-1), monocyte chemoattractant protein-1 (MCP-1), adinopectin, hemopexin and ceruloplasmin.
Patients in this trial met ACR criteria for SLE, had class III or IV LN (with or without class V overlap), a UPCR ≥1, a kidney biopsy within 12 months and evidence of active LN within three months of the baseline visit. In all, 240 patients with LN were evaluated. RAIL scores were significantly lower in patients who achieved CRR+ status; that was also the case after adjusting for confounders.
Figures 3A and 3B show the AUC for predicting renal response concurrently and at the subsequent visit. Adjusting treatment in non-responding LN patients using non-invasive methods represents an ideal clinical situation.
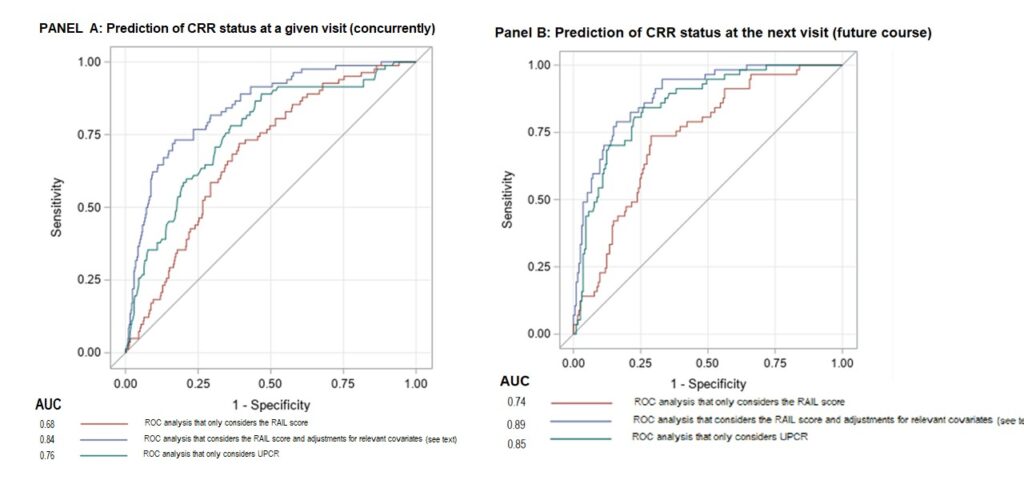
Figures 3A & 3B: ROC Curves for Detecting CRR Status Concurrently at a Visit (Panel A) & at a Subsequent Visit (Panel B), with & without Adjustment for Covariates, along with UPCR Are Depicted
Taken together, the data from these two abstracts are quite promising because, by non-invasive means, patients who will respond to treatment can be followed over time to determine their likelihood of achieving a partial or complete clinical response or, on the other hand, if they have persistent, active kidney disease, treatment adjustments can be implemented.
Revising the Damage Index
Abstract 2375: Kundacky B. et al.9
Damage in patients with SLE has been explored and documented since the mid- 1990s when the SLICC (Systemic Lupus International Cooperating Clinics) group developed an instrument, the SLICC Damage Index or SDI, to assess it. The SDI was endorsed by the ACR.10 On these bases, features associated with or predictive of damage occurrence, as well as their impact on the individual’s health as a predictor of further damage, a diminished health-related quality of life (HRQoL), increased costs and earlier mortality from cohorts and registries from around the world, have been published.11-16
This index as it stands now, however, is not consistent with how medicine is practiced now. Thus, the SLICC group decided to revise it. To this end, this group invited experts, other professionals, professional organizations and patients to participate. The theoretical framework for this process has already been published and some data were presented earlier this year.17,18 The revised index will have 45 items selected after an extensive literature review and an item-reduction process using a Delphi method (see Figure 4).
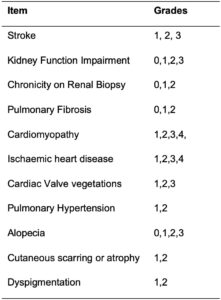
This abstract deals with how to grade or stage some of the SDI items; 11 (24.4%) of the 45 items have been chosen for staging, as noted in Table 1. This will result in a more detailed and clinically relevant assessment of organ damage in SLE patients. This revised instrument will require validation in different cohorts and registries around the world. Its application in low–middle income countries (LMIC) and whether or not it can be used as an outcome measure in clinical trials are also being considered, but these points are yet to be defined.
Treatment in SLE Falls Short of New EULAR Guidelines
Abstract 1532: Yarnall et al. & Abstract 2409: Curtis J et al.19,20
In 2023, EULAR updated its guidelines for the management of patients with SLE.21 Whether these guidelines are being followed has not been explored until now.
At this meeting, investigators took two different approaches to assess compliance. The first group assessed compliance with these guidelines by asking practicing U.S. and European (E.U.) rheumatologists how they were adhering to each of the three EULAR recommendations, and the second group established a priori what they considered suboptimal treatment.19,20
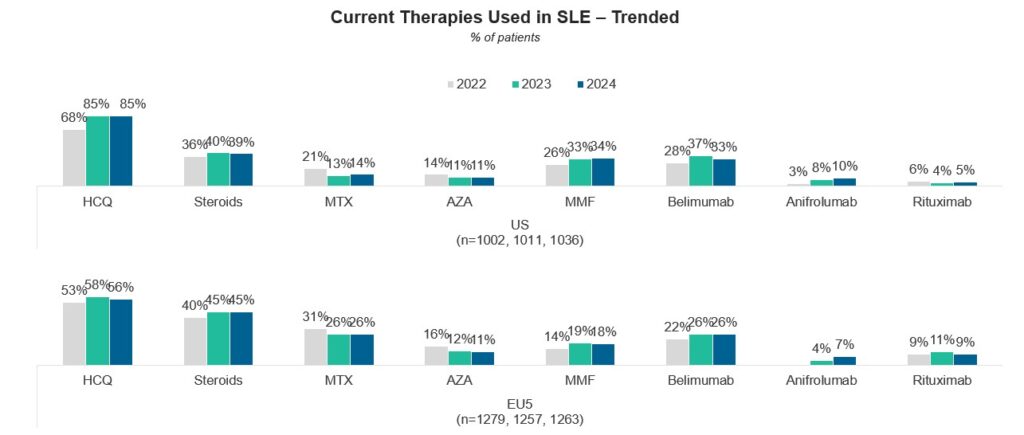
The first group of investigators reviewed the medical records of 1,263 patients with moderately/severe (M/S) SLE being treated by 265 E.U. rheumatologists, and 1,036 M/S SLE patients being treated by 176 U.S. rheumatologists; the specialists were contacted online. They found that for EULAR recommendation 1 (i.e., Use hydroxychloroquine [HCQ] in all patients unless absolutely contraindicated), U.S. rheumatologists tended to use HCQ more often than their European counterparts.
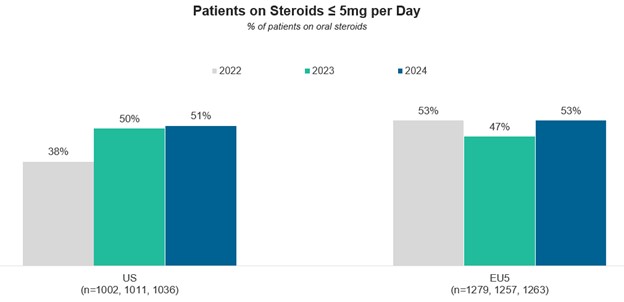
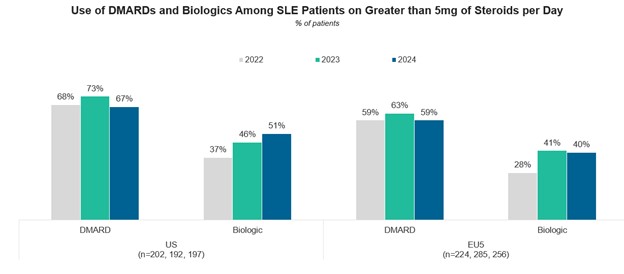
For recommendation 2 (i.e., The maintenance dose of glucocorticoids [GCs] should be ≤5 mg of prednisone), the proportion of patients receiving GCs increased from 2022 to 2023—to 39% in the U.S. and 45% in Europe; only about one-half of the patients in both groups achieved the maintenance dose of ≤5 mg of prednisone.
Finally, regarding recommendation 3 (i.e., Use additional immunosuppressive medications traditional disease-modifying anti-rheumatic drugs [DMARDs] and/or biologics in patients for whom this dose of prednisone cannot be achieved), these investigators observed a decline in the use of methotrexate and azathioprine vs. an increase in the use of biological agents in both study places.
A comparison between these data for the years 2022, 2023 and 2024 is depicted in Figures 5A–5C. For current therapies used in SLE (top part), for patients on GCs (prednisone) ≤5 mg/day (middle part), and for the use of DMARDs and biologics (bottom part).
As to the second abstract, these investigators defined what suboptimal treatment in lupus consisted of:19 1) A main dose of oral corticosteroids (OCS) as prednisone >5 mg/day for eight weeks or longer, and 2) two or more hospitalizations and/or two or more emergency department visits in the most recent 12 months covered by the claims data.
Information from more than 5,500 patients was examined using extensive clinical and pharmaceutical data. A substantial proportion of patients (34.0%) were in the suboptimal category; nearly 60% failing rule 1 (OCS), 23% failing rule 2 (hospitalizations and/or emergency department visits), and nearly 18% failing both. These figures were even higher for non-white patients and for those covered by Medicaid and Medicare.
These data, along with the one from the first commented abstract, should be taken very seriously because, despite the advances made over the years to sort out the short- and long-term adverse effects, GCs continue to be used at larger doses and for longer periods of time than the ones recommended.
These results are sobering given that they encompass only E.U. and U.S. rheumatology practices/practitioners; how much worse this could be if data from other practices and practitioners were examined cannot be inferred from these data, but chances are that they would be even higher. Of note, rheumatologists in LMICs may depend more on GCs for disease control in their patients because these agents are rather inexpensive there.
We hope that in the near future, our rheumatologists will better follow these guidelines as well as those from the ACR. Of note, at this meeting the 2024 ACR guideline for the treatment of lupus nephritis was presented.22 Largely, the recommendations reiterate the points made and subscribed to by EULAR, emphasizing the early use of triple therapy (GCs, mycophenolate acid analogs and either belimumab or a calcineurin inhibitor). An ACR guideline for the management of lupus overall is expected in 2025.
Hydroxychloroquine Adherence & Follow-up
Abstract 1540: LiCalzi et al. & Abstract 0674: Shahid S. et al.23,24
Over the past 15 years, the role of HCQ for the treatment of lupus has taken center stage; it has been shown to reduce flare-ups, improve disease activity and increase patients’ survival.25-30 It has also been shown that patients with adequate HCQ serum levels are less likely to necessitate acute care than those with lower levels.31 At this meeting, two abstracts dealt with the use of this compound in terms of adherence by those patients being prescribed with it and compliance with current guidelines by those prescribing it.23,24
The question posited in the first abstract is whether adherence to this treatment can be better documented by measuring serum HCQ levels than by self-reporting. Of nearly 8,000 patients with SLE included in this study, about 50% had an active HCQ prescription; of them, 281 patients had HCQ values available. Nearly a quarter of these patients were non-adherent, with about two-thirds having undetectable HCQ level values. Of note, there was a significant increase in ordering HCQ serum levels over time: from 0% in 2018–2020, through 0.3% in 2021 and 31.0% in 2022, to 68.7% in 2023; this is quite encouraging.
These data have significant clinical implications: Self-report is clearly not an adequate resource to judge HCQ adherence, and medication changes based on a supposed lack of response to HCQ in patients reporting to be taking it when in fact they are not, may have detrimental consequences.
Unfortunately, obtaining periodic HCQ levels in all SLE patients taking it is not feasible in most LMICs, a reality that places the burden on the patients to report their exact adherence levels, and on the physicians to assess a therapeutic response based on reports that may not be accurate.
In the second abstract, the authors examined whether the current guidelines for monitoring ocular toxicity in patients taking this compound were being followed.24 These investigators examined the degree to which ophthalmologic evaluations as recommended by the American Academy of Ophthalmology were being followed.32
In a U.S. academic clinic, medical records were reviewed for 227 patients with lupus who were taking HCQ, according to medical and pharmacy records, from 2016 to 2023 and who had five yearly visits. Only 35% of the patients had an evaluation at treatment initiation and only about 12% had an evaluation within the ensuing five years.
These data are of great concern because ocular toxicity is a well-recognized complication of HCQ treatment. We owe it to our patients to be more compliant with these recommendations. It will be of interest to assess compliance with these guidelines in other settings and geographies. I suspect they are not any better and, perhaps, even worse.
CD19-CAR-T Cell Therapy
Abstract 1749: Hagen et al.33
Current treatments for systemic autoimmune disease may mitigate to some extent the inflammatory-autoimmune processes that characterize them, without altering the ongoing immune process. Treatments aimed at significantly ameliorating or stopping these disease processes are, therefore, welcomed. One such modality is the use of genetically modified T cells to produce a chimeric antigen receptor-T cell or CAR-T cell therapy; such treatment strategy provides T cells with the ability to target specific antigens because the receptors combine both antigen-binding and T cell-activating function into a single chimeric receptor.
The process of obtaining these cells is cumbersome; it first requires apheresis and then transfection of the T cells with the necessary DNA coding to produce a new receptor capable of recognizing the target antigen (e.g., CD19). This is followed by leukodepletion to allow space for T cell expansion; finally, CAR-T cells are reinfused, so the innate signaling can turn on the subject’s machinery to induce the destruction of B cells.
During this process, patients are hospitalized to monitor for the possible occurrence of cytokine release syndrome (CRS), a condition that can be very serious.
CAR-T cell-based therapy was approved in the U.S. in 2017; since then, it has been used for the treatment of adult and children with some hematologic malignancies in selected centers.34,35 Its use in the treatment of lupus and other autoimmune diseases has recently been reported with encouraging results.36-39
The data presented in this report correspond to 30 patients with diverse systemic autoimmune diseases—18 with SLE, five with immune-mediated myopathy (IMM) and seven with systemic sclerosis (SSc)—who had been previously refractory to multiple treatment modalities. Treatment responses were recorded, as well as side effects (CRS, immune effector cell-associated neurotoxicity syndrome [ICANS] and immune effector cell-associated hematotoxicity syndrome [ICATS]). Follow-up of six or more months was possible in 25 of the patients.
All patients with achieved remission according to DORIS, as did all patients with IMM (2016 ACR/EULAR), and no progression of interstitial lung disease occurred in any patient with SSc. One IMM patient relapsed after being on remission and on no medications for 15 months. Reported side effects included mild CRS in two patients, but none developed either ICANS or ICATS. In short, these data taken together with other recently published reports are quite encouraging. However, at least at the present time, this therapy can only be used in highly specialized centers around the world—those in possession of the needed physical and professional infrastructural capabilities.
Assessing the Cost of SLE
Abstract 2682: Clarke AE et al.40
In most economic studies of SLE, only direct costs are considered; indirect costs represented, for example, by losses in productivity by unpaid labor are usually not factored in. In this study, investigators from six Canadian centers studied patients with SLE fulfilling SLICC criteria. Total indirect costs were examined using a validated questionnaire on lost productivity, which included the following factors: 1) absenteeism (i.e., time lost from paid labor because of illness); 2) presenteeism (i.e., degree of productivity impairment in paid/unpaid labor, based on a visual analog scale); and 3) opportunity costs (i.e., additional time patients would be working in paid/unpaid labor if not ill).
These costs were derived by comparing the time patients were working vs. a matched (by age, gender, geographical area) group of subjects from the general population. Indirect costs were derived from age- and sex-specific wages for Canada.
In all, 1,804 SLE patients were studied; their data and the costs analysis are depicted in Figures 6A and 6B. As can be appreciated, the total indirect costs amounted to over $36,000 (Can.) for women, and to over $35,000 (Can.) for men. Overall, women incurred higher opportunity costs from unpaid labor, but had lower opportunity costs for paid labor.
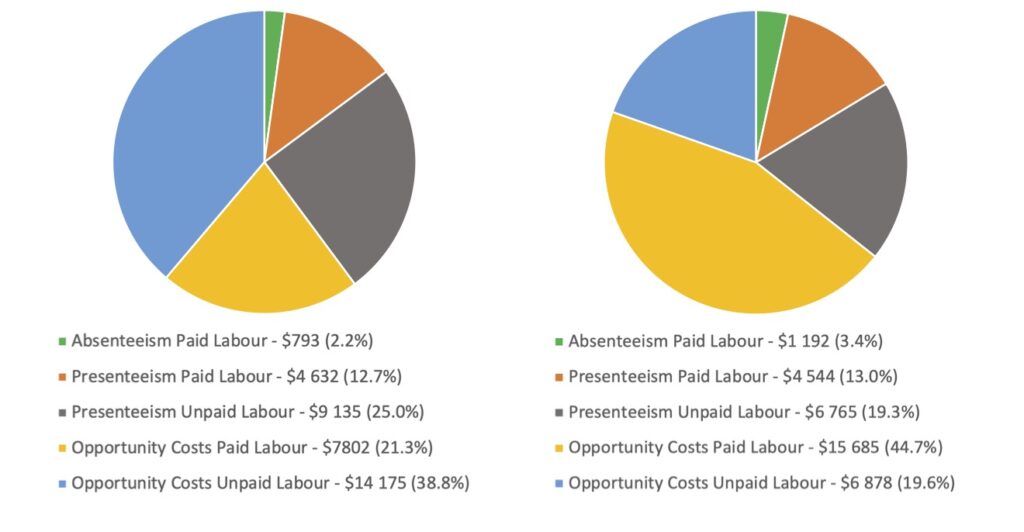
Figure 6A (left): Components of Annual Indirect Costs: Female | Total $36,538 (2023 Canadian Dollars); Figure 6B (right): Components of Annual Indirect Costs: Male | Total $35,064 (2023 Canadian Dollars)
These data emphasize the need to consider indirect costs in the assessment of the economic impact of lupus. Given the fact that SLE affects primarily women in the prime of their life, these data are of significant relevance. Indirect costs should, thus, be included in future economy-related SLE studies.
Graciela S. Alarcón, MD, MPH, MACR, is the Jane Knight Lowe Chair of Medicine in Rheumatology (Emeritus), Department of Medicine, The University of Alabama at Birmingham, Marnix E. Heersink School of Medicine, Birmingham, Ala., and professor emeritus, Department of Medicine, School of Medicine, Universidad Peruana Cayetano Heredia, Lima, Perú.
References
- Markus H, Khunsriraksakul C, Foulke G, et al. Utilizing electronic health records to identify clinical features of ANA-positive patients imparting high risk for progression to systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Choi M, Moghaddam F, Sajadi M, et al. Rheumatology diagnostics utilizing artificial intelligence (ANA Reader) for ANA pattern identification and titer quantification [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Rogers J, Clowse M, Pisetsky D, et al. The influence of trauma on features of type 2 SLE [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Pisetsky DS, Clowse MEB, Criscione-Shreiber LG, et al. A novel system to categorize the symptoms of systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2019 Jun;71(6):735–741.
- Eudy AM, Clowse ME, Corneli A, et al. The type 1 and 2 systemic lupus erythematosus model: Perspectives of people living with systemic lupus erythematosus. Lupus. 2024 Mar;33(3):266–272.
- Schunurr PP, Spiro A III, Vielhauer MJ, et al. Trauma in the lives of older men: Findings from the Normative Aging Study. Journal of Clinical Geropsychology. 2002;8:175–187.
- Fava A, Concoff A, O’Malley T, et al. A urinary biomarker panel to predict the probability of histologically active lupus nephritis [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Gomez E, Goldman D, Paz M, Petri M, Andrade F. Autoantibodies to transcription factor a mitochondria are associated with damage accrual, malignancy risk and mortality in SLE [abstract]. Arthritis Rheumatol. 2024;76(suppl 9). Abstract 1684
- Kundakci B, Barber M, Clarke A, et al.; Organ Damage Index (SDI) collaborators O. Can damage items be graded according to severity in a revised organ damage index for lupus? [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematous. Arthritis Rheum. 1996;39:363–369.
- Rahman P, Gladman DD, Urowitz MB, et al. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus. 2001;10(2):93–96.
- Cardoso CR, Signorelli FV, et al. Initial and accrued damage as predictors of mortality in Brazilian patients with systemic lupus erythematosus: A cohort study. Lupus. 2008 Nov;17(11):1042–1048.
- Alarcón GS, Roseman JM, McGwin G Jr., et al. Systemic lupus erythematosus in three ethnic groups. XX. Damage as predictor of further damage. Rheumatology (Oxford). 2004 Feb;43(2):202–205.
- Barber MRW, Hanly JG, Su L, et al. Economic evaluation of damage accrual in an international systemic lupus erythematosus inception cohort using a multistate model approach. Arthritis Care Res (Hoboken). 2020 Dec;72(12):1800–1808.
- Wang C, Mayo NE, Fortin PR. The relationship between health-related quality of life and disease activity and damage in systemic lupus erythematosus. J Rheumatol. 2001 Mar;28(3):525–532.
- Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in systemic lupus erythematosus: Results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015 Sep;74(9):1706–1713.
- Johnson SR, Gladman DD, Brunner HI, et al. Evaluating the construct of damage in systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2023 May;75(5):998–1006.
- Kundakci B, Barber M, Clarke AE, et al. Lupus damage index revision—Item generation and reduction phase [Abstract POS1139]. Ann Rheum Dis. 2024;83(Supp 1).
- Yarnall M, Rex R, May S. Not yet on track: Retrospective chart analysis reveals physician’s treatment approaches in SLE fall short of new EULAR guidance [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Curtis J, Holladay E, Mudano A, et al. Adherence to EULAR recommendations and sub optimal management of systemic lupus erythematosus in a network of community-based rheumatology practices in the United States [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Fanouriakis A, Kostopoulou M, Andersen J, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2024 Jan 2;83(1):15–29.
- 18M17: 2024 Updated ACR Guideline for the Diagnosis and Treatment of Lupus Nephritis. Session at ACR Convergence 2024. 2024 Nov 18. [Guideline summary.]
- LiCalzi M, Koumpouras F, Renaldi J. Project HOPE: HydrOxychloroquine Adherence ProjEct [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Shahid S, Barrett T, Manocha S, Bichile T. Hydroxychloroquine screening adherence: Insights from Highmark claims data [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Alarcón GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: Data fromLUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. 2007 Sep;66(9)1168–1172.
- Shinjo SK, Bonfá E, Wojdyla D, et al. Antimalarial treatment may have a time-dependent effect on lupus survival: Data from a multinational Latin American inception cohort. Arthritis Rheum. 2010 Mar;62(3):855–862.
- Nguyen Y, Blanchet B, Urowitz MB, et al. Association between severe non-adherence to hydroxychloroquine and systemic lupus erythematosus flares, damage, and mortality in 660 patients from the SLICC inception cohort. Arthritis Rheumatol. 2023 Dec;75(12):2195–2206.
- Hill DD, Eudy AM, Egger PJ, et al. Impact of systemic lupus erythematosus disease activity, hydroxychloroquine and NSAID on the risk of subsequent damage and death: Analysis in a single center US medical centre. Lupus Sci Med. 2021 Apr;8(1):e000446.
- Dima A, Jurcut C, Chasset F, et al. Hydroxychlroquine in systemic lupus erythematosus: Overview of current knowledge. Ther Adv Musculoskeletal. 2022 Feb 14;14:1759720X211073001.
- Correa-Rodriguez M, Rueda-Medina B, Callejas-Rubio J-L, et al. Adherence to antimalarials and glucocorticoids treatment and its association with self-reported disease activity in systemic lupus erythematosus. Lupus. 2023 Jan;32(1):74–82.
- Garg S, Castor BA, Saric C, et al. Therapeutic hydroxychloroquine blood levels are associate with fewer hospitalizations and possible reduction of health disparities. Arthritis Care Res (Hoboken). 2024;Aug 26. doi:10.1002/acr.25422.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016 Jun;123(6):1386–1394.
- Hagen M, Müller F, Wirsching A, Tur C, et al. Safety and long-term efficacy of CD19-CAR T-cell therapy in 30 patients with autoimmune disease [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).
- Haslauer T, Greil R, Zaborsky N, Geisberger R. CAR T cell therapy in hematological malignancies. Int J Mol Sci. 2021 Aug 20;22(16):8996.
- Zhang X, Zhang H, Lan H et al. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. 2023 Feb 20;14:1101495.
- MacKensen A, Müller F, Mougiakakos D, et al. CAR-T cell therapy in autoimmune diseases. Lancet. 2023 Nov 25;402(10416):2034–2044.
- MacKensen A, Müller F, Mougiakakos D, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022 Oct;28(10):2124–2132.
- Müller F, Taubman J, Bucci L, et al. CD19 CAR T-cell therapy in autoimmune disease—A case series with follow up. N Eng J Med. 2024 Feb 22;390(8):687–700.
- Howard JF Jr., Vu T, Mozaffar T. CAR T-cell therapy in autoimmune diseases. N Eng J Med. 2024 May 2;390(17):1629–1631.
- Clarke A, St-Pierre Y, Barber M, et al. The forgotten costs of SLE: Estimating indirect costs in a national SLE cohort [abstract]. Arthritis Rheumatol. 2024;76(suppl 9).