(Click for larger image)
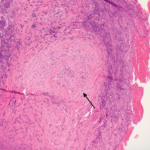
Figure 1: Hematoxylin & Eosin Staining
Enlarged, hypercellular and lobular-appearing glomeruli; capillary loops are thickened and poorly defined (arrow). The mesangium is moderately expanded by extracellular matrix and increased numbers of mononuclear cells (star).
A 35-year-old female with a history of systemic lupus erythematosus (SLE) without kidney involvement was admitted to our hospital with low-grade fevers, headache, increasing lower extremity edema and elevated blood pressure.
History
She was first diagnosed with SLE as a teenager when she developed oral ulcers and pleuritic chest pain and tested positive for anti-Smith and anti-nuclear antibodies (ANAs), meeting four of 11 ACR criteria for SLE. As an adult, her SLE recurred every few years, manifesting as oral ulcers, alopecia, arthritis, biopsy-proven leukocytoclastic vasculitis (LCV) over her lower extremities and hypocomplementemia. She had no prior episodes of nephritis.
Until two months prior to presentation, her symptoms were well controlled with hydroxychloroquine, mycophenolate mofetil (MMF) and low-dose prednisone. However, while transitioning between rheumatologists, she stopped her medications and developed her usual SLE symptoms, including oral ulcers, arthritis and lower extremity rash. In addition, she reported low-grade fevers, headaches, fatigue, pleuritic chest pain, dyspnea on exertion, six-pillow orthopnea and pitting edema in her lower extremities. A physician family member prescribed 60 mg of prednisone daily. Her breathing and chest pain improved, but she continued to experience headaches and recorded her own blood pressures, which reached systolic blood pressure of 225 mmHg.
Her new rheumatologist found hypocomplementemia and proteinuria, hydroxychloroquine was resumed, MMF changed to azathioprine, and lisinopril was prescribed. Over the next several weeks, her symptoms improved, but hypocomplementemia and proteinuria continued. Her nephrologist scheduled an elective kidney biopsy, but prior to her biopsy low-grade fevers, headache, pitting edema and hypertension recurred. She sought emergency care resulting in admission to our institution.
Hospital Assessment
On initial assessment, she was afebrile with a blood pressure of 170/90 mmHg, breathing comfortably with an oxygen saturation of 97% on room air. She was in no distress and had moderate periorbital edema. Her lungs were clear to auscultation. Examination of her lower extremities revealed synovitis in her left ankle and bilateral lower extremity pitting edema with an overlying palpable purpuric rash with a few shallow ulcers.
Her basic metabolic profile was within normal limits and included a BUN of 22 mg/dL and creatinine of 0.83 mg/dL. Her complete blood cell count was remarkable for a low, but stable hematocrit at 27% (lower limit of normal 36%). ESR and CRP were elevated at 27 mm/hr and 4.4 mg/L, respectively. Serologies yielded a positive ANA at 1:640 (speckled), while the remainder of the extractable nuclear and double-stranded DNA autoantibodies was negative. Complement levels were low, with C3 at 52 and C4 at 2 mg/dL, respectively (normal C3 90–180, C4 10–40 mg/dL). Her urine contained 3+ blood, 3+ protein and hyaline casts. The spot urine protein to creatinine ratio was 328:51, suggesting 6.4 g of urinary protein excretion over 24 hours. Antiphospholipid antibodies were negative, while ANCA and cryoglobulins were pending.
(Click for larger image)
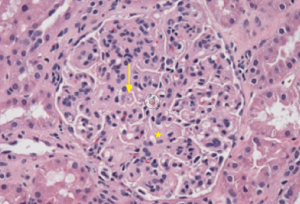
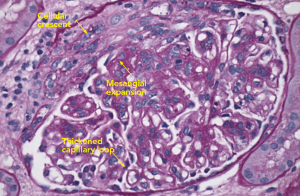
Figure 2: PAS Staining
Example of a glomerulus that contains a focal cellular crescent and expanded mesangium. Thickened capillary loops suggest double contouring of the basement membrane (see Electron Microscopy, Figure 4).
Due to her history of SLE, elevated blood pressure and new-onset nephrotic range proteinuria, the nephrology service performed a kidney biopsy early in her admission to determine if she had developed lupus nephritis. Light microscopy of the kidney biopsy revealed multiple glomeruli containing mesangial expansion and cellular crescents (see Figure 1). Immunofluorescence staining demonstrated faint, granular deposits of IgG and IgM across glomeruli, and C3 along capillary walls (see Figure 2).
In general, lupus nephritis kidney biopsies can exhibit mesangial expansion and cellular crescents, along with strong immunofluorescence staining for IgG, IgM, IgA, C3, C1q, which is known as a “full house.” In this case, the presence of faint granular immunofluorescence and cellular crescents suggested another diagnosis, like ANCA-associated pauci-immune kidney vasculitis.
Approximately 15–20% of SLE patients test positive for ANCA, although the clinical significance is not clear.1 Some data suggest that positive ANCA testing in lupus patients is associated with nephritis or an overlap syndrome, consisting of lupus nephritis and pauci-immune crescentic glomerulonephritis, which is rare and found in case reports.2 In this patient, the initial biopsy findings suggested an ANCA-associated pauci-immune kidney vasculitis, so she was empirically treated as such. For induction, she received intravenous methylprednisolone 1 g for three days, followed by weight-based oral prednisone of 1 mg/kg. Rituximab (375 mg/m2) was administered, and to be given once weekly for four weeks. Using rituximab in this clinical setting was based on clinical trial data comparing rituximab with cyclophosphamide treatment in patients with newly diagnosed ANCA-associated kidney vasculitis (but without SLE). There was no difference in relapse, death or end-stage kidney disease at two years.3 She was discharged with a steroid taper and outpatient visits to receive rituximab.
Several days after discharge, new diagnostic information returned. Electron microscopy of the kidney biopsy revealed large subendothelial deposits with adjacent substructures and serum cryoglobulins returned at 4% (normal negative), suggesting that her initial presentation was due to cryoglobulinemia, rather than ANCA-associated pauci-immune glomerulonephritis (see Figure 3).
The most common causes of cryoglobulinemia, which included negative viral hepatitis serologies and an absent monoclonal spike on serum and urine protein electrophoresis, were ruled out. The ANCA was negative, along with negative titers for MPO and PR3.
(Click for larger image)
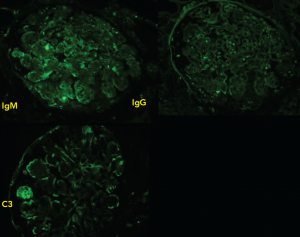
Figure 3: Immunofluorescence
Staining produces faint IgM, IgG diffusely distributed in a granular pattern, while C3 is deposited along the peripheral capillary walls.
Cryoglobulinemia
Cryoglobulinemia is classified into three types that are determined by their immunoglobulin profile. All three exhibit overlapping symptoms, but each is associated with different underlying diseases.
Type I cryoglobulinemia is defined by an isolated monoclonal immunoglobulin. It usually presents with cutaneous ulcers, peripheral neuropathy, arthralgias and kidney involvement (membranoproliferative glomerulonephritis) and is seen with a hematological malignancy or monoclonal gammopathy of undetermined significance.4
Cryoglobulinemia Types II and III generate multiple immunoglobulin classes and are, therefore, known as the “mixed” cryoglobulinemias. Both feature immunoglobulin G, but Type II contains monoclonal rheumatoid factor, and Type III contains polyclonal rheumatoid factor. The mixed cryoglobulinemias present with constitutional symptoms, cutaneous ulcers and kidney involvement. Usually, they are associated with chronic inflammatory states, such as hepatitis C, or autoimmune disorders, such as Sjögren’s, rheumatoid arthritis or SLE.4 In a series of 122 patients with SLE, cryoglobulins were positive in 25% and associated with a higher rate of cutaneous vasculitis.5
Historically, our patient met criteria for SLE on the basis of oral ulcers, pleuritis, positive anti-Smith and ANA. However, several clinical features suggest this presentation was due to cryoglobulinemia, and not SLE activity. Leukocytoclastic vasculitis is commonly seen in cryoglobulinemia, and her complements repeatedly adopted a pattern typical in cryoglobulinemia: low C4 and mildly affected C3. Her double-stranded DNA autoantibody was negative, which is commonly seen in Class III/IV nephritis.4 Interestingly, cryoglobulinemia is not known to correlate with SLE disease activity or nephropathy.5
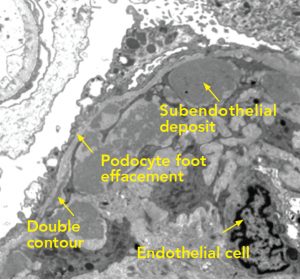
(Click for larger image)
Figure 4: Electron Microscopy
Along the periphery of the capillary loop, the podocyte foot processes are extensively effaced. Segments of the basement membrane adopt a double contour configuration. Inside the basement membrane are subendothelial deposits arranged into small microvesicles or short microtubular structures. The mesangial space is hypercellular and contains increased matrix. Endothelial cells are swollen and lack fenestrations.
2nd Thoughts
This case was educational because we were forced to reconsider the diagnosis by integrating historical clinical information with new serological and biopsy results. As this case demonstrates, SLE, cryoglobulinemia and their associated kidney diseases exhibit overlapping symptoms. Initially, we considered lupus nephritis given her history of SLE and new-onset nephrotic range proteinuria, but our view changed based on the kidney biopsy results and laboratory testing that supported cryoglobulinemia-associated kidney disease. The treatment plan with rituximab did not change, however, because there are data supporting the use of rituximab in cryoglobulinemia.
In a retrospective study of 242 cases of non-infectious cryoglobulinemia-associated vasculitis, corticosteroids and rituximab were efficacious in the treatment of the clinical, kidney and immunologic sequelae of cryoglobulinemia.6
After treatment with a single dose of rituximab and continued maintenance therapy with hydroxychloroquine, azathioprine, prednisone and lisinopril, her kidney function remains normal and her proteinuria decreased to less than 500 mg/day. Complements remained low despite treatment. Her blood pressure is well controlled, and she has had no further LCV.
Unfortunately, subsequent rituximab infusions have been delayed due to insurance coverage, and she coincidentally developed new papillary thyroid cancer, resulting in total thyroidectomy, and cervical intraepithelial neoplasia II/III for which a hysterectomy is planned. After she recovers from her hysterectomy, she will receive further rituximab infusions.
 Lindsey MacFarlane, MD, is a second-year rheumatology fellow at Brigham and Women’s Hospital. She is currently pursuing her MPH and has a research interest in osteoarthritis.
Lindsey MacFarlane, MD, is a second-year rheumatology fellow at Brigham and Women’s Hospital. She is currently pursuing her MPH and has a research interest in osteoarthritis.
 Paul Hoover, MD, PhD, is a second-year rheumatology fellow at Brigham and Women’s Hospital, starting his post-doctoral work at the Broad Institute investigating gene regulation in autoimmunity. He graduated from the Medical Scientist Training Program from Stanford University and completed his internal medicine residency at Brigham and Women’s Hospital.
Paul Hoover, MD, PhD, is a second-year rheumatology fellow at Brigham and Women’s Hospital, starting his post-doctoral work at the Broad Institute investigating gene regulation in autoimmunity. He graduated from the Medical Scientist Training Program from Stanford University and completed his internal medicine residency at Brigham and Women’s Hospital.
Acknowledgments
The authors would like to thank Helmut Rennke, MD, for supplying the light and electron microscopy images from the kidney biopsy and thoughtful conversations, and Elena Massarotti, MD, for proofreading the manuscript.
References
- Sen D, Isenberg DA. Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus. 2003;12(9):651–658.
- Hervier B, Hamidou M, Haroche J, et al. Systemic lupus erythematosus associated with ANCA-associated vasculitis: An overlapping syndrome? Rheumatol Int. 2012 Oct;32(10):3285–3290.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010 Jul 15;363(3):211–220.
- Hochberg MC, Silman AJ, Smolen JS, et al. (July 2014) Rheumatology, Sixth edition. St. Louis: Mosby.
- Garcia-Carrasco M, Ramos-Casals M, Cervera R, et al. Cryoglobulinemia in systemic lupus erythematosus: Prevalence and clinical characteristics in a series of 122 patients. Semin Arthritis Rheum. 2001 Apr;30(5):366–373.
- Terrier B, Krastinova E, Marie I, et al. Management of noninfectious mixed cryoglobulinemia vasculitis: Data from 242 cases included in the CryoVas survey. Blood. 2012 Jun 21;119(25):5996–6004.