Effective therapies to prevent or halt this progression will need to disrupt the cellular events illustrated in Figure 1).
Challenges in Scleroderma Treatment
The incomplete understanding of the pathogenesis of scleroderma has historically been one barrier to the development of effective treatments for the disease. However, additional challenges exist in developing new and effective treatments for patients with scleroderma (see Table 1).
The disease is heterogeneous, with some patients having severe skin involvement only and others having internal organ involvement such as ILD, PH, gastrointestinal disease or renal crisis. This heterogeneity poses challenges when trying to figure out a one-drug-fits-all approach and affects enrollment of patients in clinical trials.
Similarly, there is variability in the trajectory of the disease, with some patients having rapid progression of their disease and others remaining stable for many years. Predicting which patients may have more rapid progression of disease remains a challenge. To date, clinical outcomes in trials have focused on the modified Rodnan skin score (mRSS; a validated measure of skin thickness in 17 locations, graded on a scale of 0–3) and the forced vital capacity (FVC). New efforts are underway to develop a more comprehensive outcome measure that would incorporate multiple variables. To this end, the Composite Responder Index in diffuse cutaneous SSc (CRISS) has been developed and is awaiting validation in a clinical trial.7 This index incorporates both mRSS and FVC, as well as the physician global assessment, the patient global assessment and the Health Assessment Questionnaire-Disability Index (HAQ-DI).
Current Therapeutics
Currently, no approved targeted therapies exist for the treatment of scleroderma. Treatment strategies have focused on traditional immunosuppressive therapies that have proven efficacy in other rheumatic conditions.
The two best studied medications in the treatment of SSc, and specifically SSc-ILD, have been cyclophosphamide and mycophenolate mofetil (MMF).
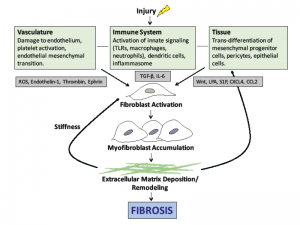
(click for larger image)
Figure 1: The pathogenesis of scleroderma. Vascular injury, immune system activation and tissue damage lead to the release of mediators that cause fibroblast activation, myofibroblast accumulation and ultimately the deposition of extracellular matrix. Note: ROS, reactive oxygen species; TLR, toll-like receptor, TGF-β, transforming growth factor beta; LPA, lysophosphatidic acid.
In the Scleroderma Lung Study I, a year of treatment with oral cyclophosphamide for SSc-ILD was found to be modestly better than placebo in stabilizing FVC at one year.8 However, in a follow-up study, this effect was reduced at two years, suggesting a transient benefit. In the recently completed Scleroderma Lung Study II, mycophenolate mofetil was compared to cyclophosphamide for the treatment of SSc-ILD.9 MMF was found to be non-inferior to cyclophosphamide with comparable improvements in FVC in both treatment groups at 24 months. Of note, the mRSS improved in both treatment arms with a trend favoring cyclophosphamide.

