Based on the results of these two trials, first-line treatment for progressive SSc‑ILD will likely shift from cyclophosphamide to MMF given its more favorable side-effect profile. MMF has separately been evaluated for the treatment of scleroderma skin disease, albeit in small cohorts, and may have beneficial effects on lowering skin score, particularly in patients with early diffuse disease.10,11
With further advances in understanding the biology of the disease, stratifying patients, & determining comprehensive outcome measures for clinical trials, it is highly likely that effective therapies for scleroderma fibrosis will emerge in the next decade.
Other traditional disease-modifying anti-rheumatic drugs (DMARDs), including methotrexate, have been evaluated in scleroderma. A small randomized, controlled trial compared one year of methotrexate with placebo in patients with early (less than three years) diffuse scleroderma.12 Despite the small size of the study, there was a trend toward improvement in the mRSS in the methotrexate group. Based on this and another smaller study, the EULAR and European Scleroderma Trial and Research Group (EUSTAR) recommend methotrexate for skin disease in patients with early diffuse cutaneous SSc (dcSSc).13,14
Rituximab, a monoclonal antibody against CD20, used in the treatment of rheumatoid arthritis and ANCA-associated vasculitis, has come of interest in the treatment of SSc. A small nested case-control study demonstrated that patients treated with rituximab vs. matched controls had improvement in their mRSS, and prevented further decline of FVC in a small subset of patients with ILD.15 Based on this and other small studies, there is hope that rituximab may be beneficial in SSc, but larger studies are needed.16
Targeted Therapeutics
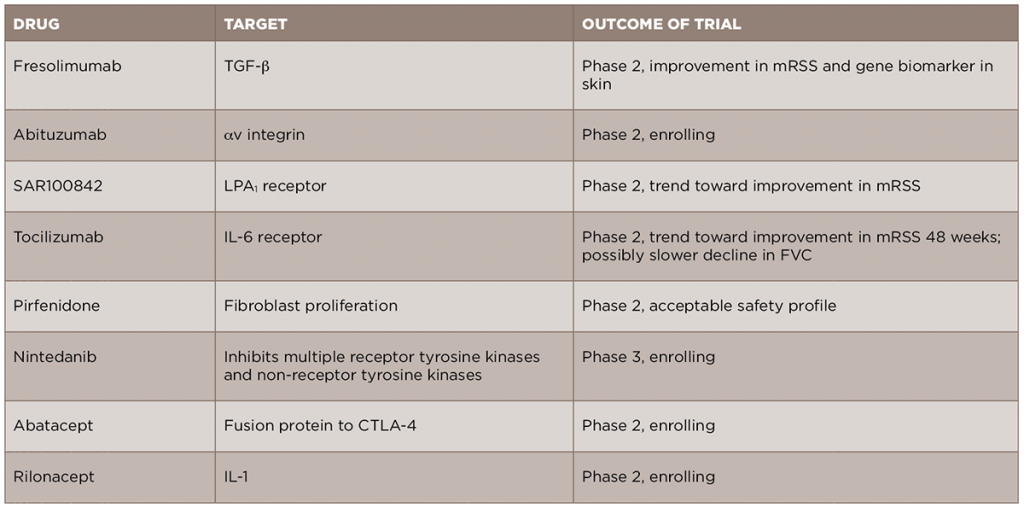
In light of the recent advances in the understanding of the pathogenesis of SSc, new anti-fibrotic treatments are currently in development and being studied for the treatment of the disease (see Table 2). We will review some of these new treatments by their molecular targets.
Given the central role of TGF-β in the development of scleroderma fibrosis, it is a natural target for therapy in SSc. A recent open-label trial of fresolimumab, a monoclonal antibody against TGF-β that neutralizes all isoforms of the molecule, compared two doses of the drug in patients with early (less than two years) dcSSc.17 The primary outcomes of this trial were change in mRSS and change in mRNA levels of two biomarkers measured in the skin, cartilage oligomeric protein (COMP) and thrombospondin-1 (THBS1), at 24 weeks. In both groups, there was a relatively rapid decrease in mRSS and in TGF-β regulated biomarker genes, in particular THBS1, suggesting that targeting TGF-β may be an effective treatment strategy. Of note, there were a number of cases of anemia in both groups, and this will need to be examined further in future, larger studies. Another drug targeting the TGF-β pathway, abituzumab, a monoclonal antibody against αv integrin, will be evaluated in an upcoming Phase 2 trial for SSc-ILD (ClinicalTrials.gov identifier: NCT02745145).