Dr. van den Berg, among others, has been instrumental in demonstrating the contribution of IL-17 to arthritis in various mouse models. In some models, with dynamic changes in the cell composition of the synovitis, he was able to show that the TNF contribution seen in early disease could be lost in a more chronic phase of the disease, replaced by the key role of IL-17.10 In a paper we published together, he also showed that the addition of IL-4 through gene therapy could inhibit IL-17 production and action and protect against bone destruction by reducing the key interactions between RANK and RANK ligand.11
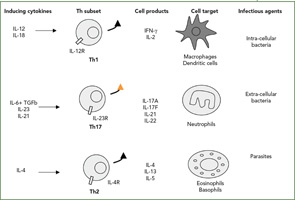
A New Subset of T cells
The Th1/Th2 classification was described by my friends Tim Mossman and Bob Kaufman, working then at Dnax in Palo Alto, Calif. Indeed, such simple dichotomy has been useful to classify both mouse and human diseases (See Figure 2, right). According to this classification, infections with intracellular pathogens such as tuberculosis (TB) are Th1 diseases, and asthma and other allergic conditions are Th2 diseases. From the beginning, however, the fixed pattern of T cell function made it difficult to understand chronic diseases. When we looked at a long list of T-cell clones from the RA synovium we had obtained from Jacob Natvig, MD, professor in the Institute of Immunology at the University of Oslo in Norway, it was clear that the IFNγ-producing cells were more common than the Th2 IL-4–producing cells.
Based on this type of result, RA was classified as a Th1 rather than a Th2 disease. When we added our bioassay for IL-17, clones could be classified more precisely. IL-17–producing cells were never found in IL-4–producing cells but could be found in IFNγ-producing cells. These results, obtained in 1999, are the first description of the production of IL-17 by a different subset of T cells.12 In the RA synovium, IL-17–producing cells have a plasma cell–like morphology (See Figure 3, p. 24).
The official description of the Th17 cells was made in 2005 in the mouse.13,14 This lineage was considered independent from the Th1 lineage. Whereas the key cytokines for the induction of IFNγ are IL-12 and IL-18, the key cytokines for the induction of IL-17 are IL-6 plus TGFβ followed by IL-23. The associated transcription factors are also different, with tBet for Th1 pathway and ROgammat for the Th17 pathway.15
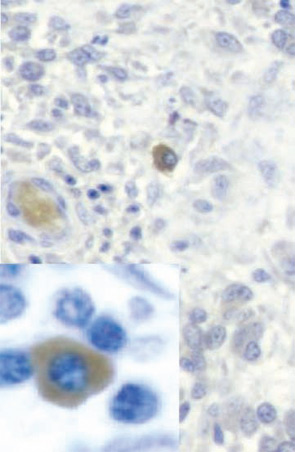
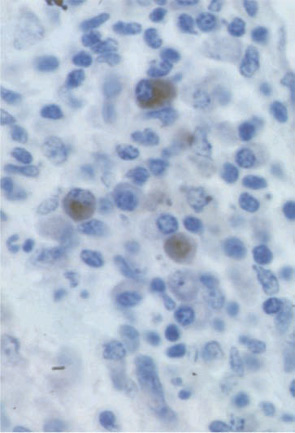
Role of IL-17 in Aspects Still Uncontrolled by TNF Inhibitors
Blocking TNF has been a major step in RA treatment and has produced dramatic improvements in patients not adequately responsive to other DMARDs. In addition to being an important advance in therapy, the efficacy of TNF blockers showed that, although the list of target cytokines is long and forever growing, blocking a single one works, probably by disrupting the synergistic interactions between cytokines. We all know that other interventions are needed to one day cure RA. It is of interest to consider if controlling IL-17 could bring us closer.
