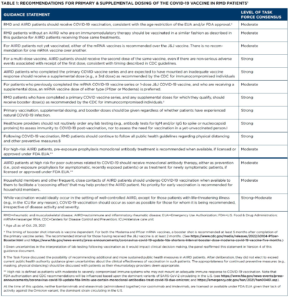
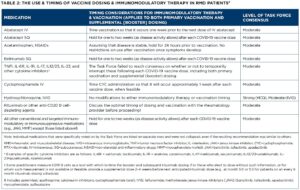
On Feb. 2, 2022, the ACR released a revised version of its COVID-19 Vaccine Clinical Guidance Summary (version 5, revised). A full paper was originally published online in Arthritis & Rheumatology (A&R) on March 17, 2021, and version 5 has been submitted to A&R for publication. Updates include:
- Recommendations differentiating supplemental and booster doses, including timing considerations; and
- Guidance on pre- and post-exposure prophylaxis with monoclonal antibody treatment, considering updated EUAs.
This document is intended to provide guidance to rheumatology providers on the use of the COVID-19 vaccine and the associated management of rheumatic and musculoskeletal disease patients around the time of vaccination against SARS-CoV-2. These statements were based upon a dearth of high-quality data and are not intended to replace clinical judgment. Modifications made to treatment plans, particularly in complex rheumatic disease patients, are highly disease, patient, geography and time specific and, therefore, must be individualized as part of a shared decision-making process. This guidance is provided as part of a living document, recognizing rapidly evolving evidence and the anticipated need for frequent updates. The target audience for this guidance is providers and patients in the U.S., although the ACR recognizes that people outside of the U.S. may read and use the information provided here.
Visit the ACR website for updates.
Notes
- Refer to Table 2 in the Guidance Summary: https://tinyurl.com/2p2amm4h
- Refer to Table 3 in the Guidance Summary: https://tinyurl.com/2p2amm4h