Patients with systemic lupus erythematosus often report severe and distressing symptoms of cognitive dysfunction, such as confusion, inability to concentrate, and forgetfulness. These symptoms are manifestations of neurological lupus which, while difficult to diagnose clinically, can be assessed by formal neuropsychological testing. In the past, although cognitive dysfunction in lupus was attributed to diffuse brain damage induced by antibodies, new imaging tools can demonstrate that brain injury can be localized and restricted anatomically to specific brain regions. Prior ideas about the pathogenesis of brain dysfunction focused on gray-matter injury. New research, however, points to white-matter injury as an increasingly likely explanation for patient symptoms.
Leaps in Brain Science
Neuropsychologists distinguish among various domains of cognitive function: attention, learning and memory, reasoning, and visuomotor skills. These domains have specific anatomic addresses in the brain, and lupus patients display distinct patterns of abnormalities in these domains. However, neurobiologists, neuropsychologists, neurologists, and rheumatologists can speak different scientific languages. Judah Denburg, MD, professor of medicine at McMaster University in Hamilton, Ontario, in 1990 and Matthew Liang, MD, MPH, professor of medicine at Harvard Medical School in Boston, in 1998 convened conferences to facilitate communication across disciplines, calling for standardized vocabularies and definitions, but controversies regarding mechanisms and treatments remain. These controversies unfortunately can limit investigation into disease pathogenesis and the development of a coherent approach to therapy.
In the 10 years since the last conference on neurological lupus, awards for research on the nervous system have abounded. The 2000 Nobel Prize was awarded to Arvid Carlsson, ML, MD, Paul Greengard, PhD, and Eric Kandel, MD, for their studies on signal transduction in the central nervous system; the 2004 Nobel Prize was awarded to Richard Axel, MD, and Linda Buck, PhD, for studies on the organization of the olfactory system; and the 2003 Nobel Prize was awarded to Paul Lauterber, PhD, and Sir Peter Mansfield, PhD, for the science underlying magnetic resonance imaging (MRI). Together, these advances have provided new tools for the study of abnormalities in cognition, as well as other aspects of higher brain function. Indeed, cognition is now an important research focus in the fields of traumatic brain injury, progressive neurological disease (such as Alzheimer’s and Parkinson’s diseases), genetic disorders (Huntington’s disease), and miscellaneous neurological (headaches and cerebral vascular disease) and medical (post–coronary artery bypass surgery, post-anesthesia) disorders.
Table 1: Neuro Vocabulary
Cognitive efficiency: The ease or proficiency of information processing skills, such as complex attention, problem solving, and reasoning, necessary for mental operations. Tests often involve the speed at which mental tasks can be done.
Cognitive dysfunction: A decline in thinking skills such as concentration, reasoning, memory, language, or perceptual skills resulting from direct or indirect effects on brain structures and systems. The rate of completion of tests is less important.
Focus on Cognition in Lupus
To apply these advances to the study of lupus, we organized a conference on cognitive dysfunction, held in New York City this April and sponsored by the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York City. The attendees of the conference were a diverse and eclectic group, and certainly polyglot in their scientific language. Cell biologists, neurobiologists (including Dr. Greengard), neuropsychologists, neurologists, imaging experts, clinicians, and “mouse doctors” who work with animal models of neurologic disease, all gathered in a New York hotel for state-of-the-art presentations on rapidly evolving science. This conference produced a lively dialogue among the participants and demonstrated how much we know and how much we don’t know about the effects of lupus on the brain.
In their lectures at the conference, neurobiologists described how the processes of creation, maintenance, and recall of normal memory have identifiable synaptic links and precise anatomic addresses within the brain. Furthermore, they showed how these processes can be experimentally manipulated at the molecular and cell level in in vitro and in vivo animal models. Importantly, these studies showed, in both animals and humans, that memories can be imaged, interrupted, or elicited. Since memory dysfunction is one of the commonest manifestations of cognitive dysfunction in lupus, understanding the neurobiology of normal and abnormal memory is an important goal.
Neuropsychologists distinguish between brain efficiency and brain function. (See Table 1, below left.) Abnormalities of efficiency are reflected primarily in white matter, while those of function are primarily reflected in gray.
A patient’s failure to perform a neuropsychological testing task results from either an inability to comprehend the task (reduced brain function)—as in patients with posterior brain injury—or from an inability to organize sequential tasks rapidly enough for successful completion (reduced brain efficiency). Testing for domains of cognition can identify patterns that imply both the anatomy and the pathophysiology of injury. The cognitive battery studies suggested by the ACR Task Force on cognitive testing (see Table 2, right) distinguishes among the neuropsychological domains, but most papers written by rheumatologists on cognitive dysfunction in lupus do not analyze patients in such detail.
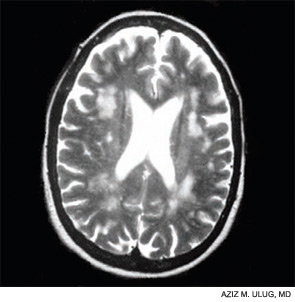
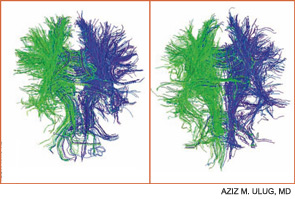
Contemporary techniques for imaging of the brain—MRI, PET, functional MRI, and diffusion tensor imaging (DTI) (see Figures 1 and 2, above)—allow the display of the anatomy of abnormal brain function and the ability to see, literally, thought in action. The images observed by these modalities do not always distinguish functional abnormalities from structural ones; also, demonstrated abnormalities may not correlate closely with cognitive function measured by formal testing. Focal breakdown of the blood–brain barrier may be critical to the localization of brain injury in diseases such as lupus, but intactness of the barrier is not easy to identify by any technology currently applicable to humans. New technologies under investigation may be more successful in defining the blood–brain barrier and may help explain the gap between the presence of autoantibodies in lupus and the occurrence focal brain changes.
Lessons from the Brain
As discussed at the conference, animal models may help define mechanisms of abnormal cognition. Mice that develop clinical lupus or are exposed to lupus autoantibodies (e.g., anti-DNA, antiphospholipid, and anti-NMDA) demonstrate behavioral changes and anatomic abnormalities that parallel those of human lupus. Time-specific disruption of the blood–brain barrier, leukoagglutination, thrombosis, microvascular injury, and autoantibody-mediated cell toxicity are plausible mechanisms of brain injury demonstrable in mice, and sometimes in men.
The Mary Kirkland Center conference’s discussion of animal and human neurobiological, behavioral, and imaging information about cognition concluded that white-matter injury (interfering with brain interconnections) may be more important in lupus than is gray-matter injury, and that immunologically driven brain injury is focal for reasons not yet understood. White-matter damage, particularly in the frontal area, can parallel neuropsychologically defined global deficits of attention and executive function, and is demonstrable on MRI or DTI. Longitudinal studies suggest that cognitive dysfunction in lupus is more likely transient and reversible than permanent.
The Mary Kirkland Center conference led to the following ideas that could inform future research: Due to the wide variability of cognitive symptoms and innate capacities among patients with lupus, longitudinal study of small numbers of individual patients may be more informative than cross-sectional studies of large patient groups. Because patients’ descriptions of cognitive symptoms are clues to anatomic localization, attention to the details of responses in neuropsychological tests offers noninvasive, inexpensive opportunities for deeper study. Finally, novel experimental cognitive tests may be specifically applicable to lupus.
Table 2. Cognitive Tests for Patients with Lupus
National Adult Reading Test (NART) (to estimate IQ)*
Digit symbol substitution test
Trail-making test (part A and B)
Stroop color and word test
California verbal learning test
Rey Osterrieth complex figure test (with delayed recall)
Wechsler Adult Intelligence Scale (WAIS) III letter numbering sequencing*
Controlled oral word association test
Animal naming
Finger tapping
* Not appropriate for use in children and adolescents.
Source: Mikdashi J, Esdaile JM, Alarcón GS, et al. Lupus. 2007;16:418-425.
The Future of Cognitive Research in Lupus
As in other disorders with white-matter pathology—such as HIV and multiple sclerosis—cognitive dysfunction in lupus may represent primarily reduced brain efficiency and not reduced brain function. Thus, continued attention to white-matter function with neuroimaging techniques such as DTI may lead to new insights into pathogenesis and approaches to therapy. Conference participants also agreed that elucidating the basis of focal lesions—such as hyperintense lesions on MRI or focal hippocampal disease—may be more informative for both diagnosis and research than the assumption that lupus cognitive dysfunction is definable by serological or cerebrospinal fluid tests. Recognizing the potential reversibility of early lesions offers hope for new treatments and improved outcomes.
In the same way that rheumatologists now distinguish different types of glomerulonephritis or skin disease in lupus, in the future we may distinguish among the many types and mechanisms of brain injury as well. Until we fully understand the meaning of cognitive dysfunction, clinicians should know that no single immunological or imaging study currently defines cognitive dysfunction in SLE. The abnormalities are real and prominent, but intelligent use of all available diagnostic tools is still required to assess a single patient.
Dr. Lockshin is director of the Barbara Volcker Center for Women and Rheumatic Disease and co-director of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery. Dr. Kozora is professor of medicine at National Jewish Health in Denver.