Years later, the development of sensitive analytic techniques to measure FMc permitted a direct test of the hypothesis. These studies indicated an inverse correlation between levels of FMc in the peripheral blood and disease activity of women with RA during pregnancy and postpartum.5 A step-by-step mechanism by which FMc could induce RA amelioration during pregnancy has recently been described.6 In the changing maternal “self” hypothesis, an important initial event was the observation that remarkably large amounts of apoptotic placental debris are released into the maternal circulation—several grams a day in the third trimester. Although fetal classical HLA molecules are not expressed at the maternal–fetal interface, the apoptotic debris likely contains fetal classical HLA molecules. Thus, amelioration of RA is thought to occur as a secondary benefit of maternal tolerance of the fetus when maternal antigen-presenting cells simultaneously present self and fetal HLA antigens, and tolerance is induced whether by T-cell anergy, deletion, or induction of T-regulatory cells.
Persistence of FMc in women many years after pregnancy was initially reported in healthy women.7 Studies were done by testing women who had sons for male DNA after sorting peripheral blood for progenitor cells; six of eight women had positive results. Most studies of FMc employ testing for male DNA in women with sons for a practical reason: chimerism in many women can be studied with the same assay.8 To address the question of whether MMc persists in a woman’s offspring, other approaches were needed. MMc had previously been found in children with immune deficiencies but whether MMc persists in immunocompetent individuals was not investigated until recently.
Using an approach in which non-transmitted maternal-specific HLA genes were identified, MMc was found to persist into adult life—even in healthy individuals.9 Results were corroborated by identifying female cells (two X-chromosome signals), among male cells (one X- and one Y-chromosome signal) in peripheral blood samples from males. The latter studies were done by fluorescence in situ hybridization with different color probes for the X and Y chromosomes. An example is shown in Figure 1. A similar approach can be used to identify male cells in female blood or organs.
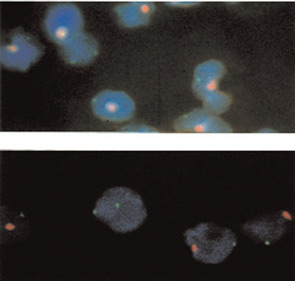
Source: J Clin Invest. 1999 Jul;104(1):41-47.
Microchimerism’s Role in Disease
The hypothesis that naturally acquired microchimerism is involved in some autoimmune disease was proposed after considering observations derived from different specialties of medicine.10 (See Figure 2) One observation was that an iatrogenic form of chimerism, graft-versus-host disease after hematopoietic cell transplantation, has many clinical similarities to autoimmune diseases—especially systemic sclerosis and also aspects of myositis and systemic lupus erythematosus.

