
The advent of sensitive molecular techniques has revealed aspects of human biology that challenge the imagination and encourage speculation about the mechanisms of disease—and microchimerism is among the most striking of these aspects. Microchimerism refers to the presence in a person or animal of a small number of cells or DNA from a genetically different individual. While unanticipated by conventional thinking, microchimerism is not an anomaly of nature. Despite beliefs on individualism and inimitability, microchimerism appears to be a part of normal biology. As now recognized, during pregnancy microchimerism occurs naturally with the exchange of cells and cell-free DNA between a mother and fetus. After birth, maternal microchimerism (MMc) is found in neonates, children, and adults. In women with prior pregnancies, fetal microchimerism (FMc) is present years later.
This remarkable situation has been investigated only recently. The extent of both short-term and long-term effects—during pregnancy and years later—are not yet known. Even a conceptual framework has not yet evolved to understand how the individual maintains health with cells of disparate origins. In this new investigative frontier, studies in rheumatologic and autoimmune diseases, including RA, systemic sclerosis, neonatal and systemic lupus, and myositis, are leading the way and generating insights with the potential for direct application from bench to bedside. This review will cover the current field of microchimerism, considering the chimeric self throughout a lifetime, in health, or when afflicted by disease.
Pregnancy Presented Initial Research Opportunities
The placenta has usually been considered a staunch barricade to keep the mother and fetus apart and prevent maternal rejection of the genetically disparate fetus. Interestingly, some earlier literature suggested otherwise, but did not gain wide acceptance.1 With the application of sensitive molecular techniques, it has become clear that maternal–fetal trafficking is in fact common. Lo and colleagues reported FMc in 100% of pregnant women and MMc in 30% of cord blood specimens.2 In these studies, testing for chimeric DNA in plasma employed sensitive quantitative PCR techniques.
More than 60 years ago, P.S. Hench observed that RA usually improves during pregnancy and recurs after delivery.3 Hench’s investigations led to the discovery of cortisol, for which he shared the Nobel Prize in 1950. Despite the tremendous importance of this discovery, subsequent work indicated that increased cortisol levels did not explain pregnancy-induced RA amelioration. Later, an immunologic explanation was proposed. According to this model, the exposure of a mother to the genetically differing fetus induces the change in maternal disease activity. As an indirect test of the hypothesis, mother–child pairs were studied for human leukocyte antigens (HLA); these studies showed that a significantly greater likelihood of RA amelioration occurred when the child was HLA class II disparate from the mother.4
Years later, the development of sensitive analytic techniques to measure FMc permitted a direct test of the hypothesis. These studies indicated an inverse correlation between levels of FMc in the peripheral blood and disease activity of women with RA during pregnancy and postpartum.5 A step-by-step mechanism by which FMc could induce RA amelioration during pregnancy has recently been described.6 In the changing maternal “self” hypothesis, an important initial event was the observation that remarkably large amounts of apoptotic placental debris are released into the maternal circulation—several grams a day in the third trimester. Although fetal classical HLA molecules are not expressed at the maternal–fetal interface, the apoptotic debris likely contains fetal classical HLA molecules. Thus, amelioration of RA is thought to occur as a secondary benefit of maternal tolerance of the fetus when maternal antigen-presenting cells simultaneously present self and fetal HLA antigens, and tolerance is induced whether by T-cell anergy, deletion, or induction of T-regulatory cells.
Persistence of FMc in women many years after pregnancy was initially reported in healthy women.7 Studies were done by testing women who had sons for male DNA after sorting peripheral blood for progenitor cells; six of eight women had positive results. Most studies of FMc employ testing for male DNA in women with sons for a practical reason: chimerism in many women can be studied with the same assay.8 To address the question of whether MMc persists in a woman’s offspring, other approaches were needed. MMc had previously been found in children with immune deficiencies but whether MMc persists in immunocompetent individuals was not investigated until recently.
Using an approach in which non-transmitted maternal-specific HLA genes were identified, MMc was found to persist into adult life—even in healthy individuals.9 Results were corroborated by identifying female cells (two X-chromosome signals), among male cells (one X- and one Y-chromosome signal) in peripheral blood samples from males. The latter studies were done by fluorescence in situ hybridization with different color probes for the X and Y chromosomes. An example is shown in Figure 1. A similar approach can be used to identify male cells in female blood or organs.
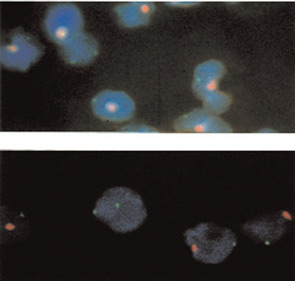
Source: J Clin Invest. 1999 Jul;104(1):41-47.
Microchimerism’s Role in Disease
The hypothesis that naturally acquired microchimerism is involved in some autoimmune disease was proposed after considering observations derived from different specialties of medicine.10 (See Figure 2) One observation was that an iatrogenic form of chimerism, graft-versus-host disease after hematopoietic cell transplantation, has many clinical similarities to autoimmune diseases—especially systemic sclerosis and also aspects of myositis and systemic lupus erythematosus.
A second observation was long-term persistence of FMc and MMc. A third observation was the predilection of autoimmune diseases in women, as well as the peak incidence of most autoimmune disease in post-reproductive years.
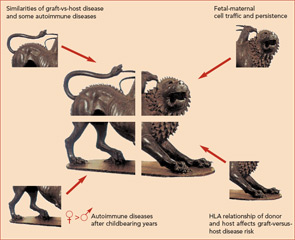
Finally, the observation that HLA relationships between a recipient and donor are critical determinants of graft-versus-host disease led to the proposal that excess HLA sharing (without complete HLA identity) might result in aberrant monitoring or interactions with microchimeric cell populations.
Microchimerism in SSc
The first report investigating microchimerism in an autoimmune disease was a prospective blinded study of FMc in women with SSc.11 FMc was measured by a quantitative test for male DNA in the peripheral blood of women who had sons. Significantly higher FMc levels were found in women with SSc compared with healthy women. Subsequent studies identified FMc in tissues of SSc patients.8 MMc has also been investigated in SSc. Unlike FMc the quantitative levels of MMc in SSc patients did not differ significantly from controls, although the prevalence (any positive result) was greater in SSc patients than controls.12 A theme that emerged from the beginning of studies of microchimerism was the importance of quantitative techniques—especially because both FMc and MMc are often found in healthy individuals without autoimmune disease but usually at low levels.
As the goal for microchimerism studies of SSc is to generate new therapeutic approaches for this difficult disease, the next question to address is how might naturally acquired microchimerism contribute to SSc? Considering an analogy with graft-versus-host disease where donor T lymphocytes are thought to mediate disease, if fetal T cells were implicated in disease pathogenesis, these cells could be selectively targeted. However, this explanation is unlikely both because of radical differences in the extent of chimerism in the two disorders and because FMc is found among T cells in healthy individuals as well as SSc patients. Indeed both FMc and MMc are found in T and B cells, monocyte/macrophages, and NK cells.13
A more likely analogy with SSc is the situation that occurs in chronic organ graft rejection. In this setting, recipient cells react with other recipient cells but aberrant immunity occurs because fragments of HLA molecules from the chimeric donor cells are being presented for immune recognition. In this case, a future therapeutic approach could be HLA peptide inhibitors that block the interaction between antigen presenting cell and T lymphocyte or compete with foreign peptides to induce tolerance rather than stimulate the T cell. Another area of active investigation in autoimmunity is the role of T regulatory cells. Important in limiting autoreactive T cells, T regulatory cells are increased in the mother during pregnancy and in cord blood and may be important in tolerance to FMc after pregnancy. If aberrant T regulatory function is found, a deficit could potentially be restored by adoptive cellular therapy.
Microchimerism in Juvenile Myositis
In studies of patients with juvenile dermatomyositis and idiopathic myositis, MMc was found in muscle biopsies but was infrequent in muscle biopsies from other diseases.14,15 Studies were performed by selecting male patients and identifying female cells in the biopsy samples. The study of dermatomyositis also found that patients had MMc in peripheral blood significantly more often than their unaffected siblings.14 Peripheral blood studies were performed by DNA testing for maternal-specific, non-shared HLA. In further studies, chimeric maternal T lymphocytes isolated from dermatomyositis patients reacted to the patient’s cells, producing interferon-g in vitro, but chimeric maternal cells from unaffected siblings did not react to the sibling’s cells.16 Thus, the investigators proposed that chimeric maternal T lymphocytes are effectors of disease in myositis patients.
Should these findings be confirmed, a potential therapeutic strategy might be to selectively deplete maternal T cells, for example, by targeted specific antibody or by apheresis. However, it is possible that targeting MMc could have adverse consequences because the role of MMc in normal biology is not yet understood and MMc is common in healthy individuals, including within T lymphocytes.12,13
Microchimerism in Neonatal Lupus Syndrome
Findings from research on stem cell biology have led to the intriguing question of whether stem cells (naturally acquired as microchimerism from pregnancy) differentiate into somatic cells. Akin to a good mystery novel in which an unexpected twist appears as the story develops, studies of neonatal lupus syndrome found that MMc can differentiate into cardiac myocytes. Neonatal lupus syndrome is a passively acquired autoimmune disease in the fetus, and maternal autoantibodies are strongly implicated in the pathogenesis. Mothers of infants with neonatal lupus syndrome have antibodies to Ro and La (SSA and SSB) but are often themselves disease-free. The most serious complication of neonatal lupus syndrome is life-threatening inflammation of the atrial–ventricular node leading to congenital heart block.
Female cells were found at autopsy in the heart tissue of boys with neonatal lupus syndrome and heart block, but were rare in age-similar controls who died from other causes. Intriguingly, more than 80% of the maternal cells expressed sarcomeric a-actin, a specific marker for cardiac myocytes.17 This interesting observation raises the question of whether “autoimmunity” sometimes occurs due to loss of tolerance to tissue-specific microchimerism. If so a different therapeutic approach might be considered, directed to restoring or promoting tissue-specific tolerance of chimerism. However, it is not yet known whether MMc is a target for immune reactions or whether MMc traveled to the heart as a secondary event in efforts to restore and regenerate disease-affected tissues.
Analogous to the above studies of MMc, FMc that apparently differentiated into tissue-specific cells has been described in other organs. In a study of seven women with various diseases—including malignancies and autoimmunity—the thyroid, cervix, intestine, and gallbladder had male cells that expressed epithelial cell markers, and a liver biopsy from one woman had male hepatocytes (all women had sons).18 As with studies of MMc, these observations suggest engraftment of multipotent microchimeric stem cells. In another report, male hepatocytes were found in liver biopsies from one-quarter of 28 women with primary biliary cirrhosis and other liver diseases—all of whom had sons.19
Thus, recent findings support the conclusion that both MMc and FMc can differentiate into somatic tissues. Therefore naturally acquired microchimerism from pregnancy could sometimes become a target for an immune response and/or could potentially contribute to tissue regeneration. If the former, new therapeutic options could block immune responses; if the latter microchimerism could potentially be exploited for therapeutic benefit in tissue regeneration.
Microchimerism in SLE
A strong rationale is provided for investigation of microchimerism in systemic lupus erythematosus (SLE) based on experimental models—especially MMc. A well-recognized murine model of SLE is created by the introduction of parental cells into non-irradiated F1 progeny and results in IgG antinuclear antibody production and fatal immune complex glomerulonephritis.20 Additionally, chronic graft-versus-host disease after allogeneic stem cell transplantation sometimes exhibits SLE-like features.
Although no study has tested for MMc in human SLE, an increased frequency of mother–child HLA class II sharing was found in a study of men with SLE.21 Male patients were selected to avoid potential confounding in women due to FMc. A few studies have tested for male DNA as a measure of FMc in peripheral blood from SLE patients.22 In a recent report male cells were found significantly more often in kidney biopsies from 49 women with lupus nephritis than control kidneys.23 Chimeric T lymphocytes as well as cells with stem cell markers were identified in some lupus nephritis specimens. Thus, experimental models and initial studies indicate further investigation is needed of microchimerism in SLE.
Summary
Bi-directional cell trafficking occurs between a mother and fetus during pregnancy. Many years after the physical union of mother and child is severed, MMc is found in her progeny and FMc is found in women who were previously pregnant. In some autoimmune rheumatologic diseases, microchimerism may be advantageous; in others, detrimental. On the beneficial side, it is likely that genetically disparate FMc contributes to the pregnancy-induced amelioration of RA. On the detrimental side, persisting FMc has been implicated in SSc pathogenesis. While only women are subject to FMc, all individuals are subject to MMc—including men, children, and nulligravid women. MMc is acquired during fetal life when the immune system is under development, whereas when FMc is acquired the immune system is mature. Therefore effects may differ.
Studies of MMc suggest that maternal effector cells could be involved in juvenile myositis and that maternal cardiac myocytes could be the immunologic targets in neonatal lupus syndrome. In addition to FMc and MMc, future studies must investigate other potential sources of naturally acquired microchimerism, such as microchimerism from an older sibling transferred via the maternal circulation or from twin–twin transfer in utero, including from an unrecognized twin lost early in gestation. Studies of rheumatologic autoimmune diseases are leading the way in this investigative frontier and generating new insights into the chimeric self in health and autoimmune disease as well as pointing the way to new possibilities in therapeutic approaches to autoimmune diseases.
Dr. Nelson is professor of immunogenetics in the clinical research division of Fred Hutchinson Cancer Research Center and professor of medicine at the University of Washington in Seattle. Dr. Stevens is assistant professor of pediatrics in the division of immunology and rheumatology at the University of Washington in Seattle and in the department of immunology and rheumatology at Children’s Hospital and Regional Medical Center.
References
- Tuffrey M, Bishun NP, Barnes RD. Porosity of the mouse placenta to maternal cells. Nature 1969;221:1029-1030.
- Lo YMD, Lau TK, Chan LYS, Leung TN, Chang AMZ. Quantitative analysis of the bi-directional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem. 2000;46:1301-1309.
- Hench PS. The ameliorating effect of pregnancy on chronic atrophic (infectious rheumatoid) arthritis, fibrositis, and intermittent hydrarthrosis. Mayo Clin Proc. 1938;13:161-167.
- Nelson JL, Hughes KA, Smith AG, et al. Maternal-fetal disparity in HLA class II alloantigens and the pregnancy-induced amelioration of rheumatoid arthritis. N Engl J Med. 1993;329:466-471.
- Yan Z, Lambert NC, Guthrie KA, et al. Male microchimerism in women without sons: Quantitative assessment and correlation with pregnancy history. Am J Med. 2005;118:899-906.
- Adams K, Yan Z, Stevens AM, Nelson JL. The changing maternal “self” hypothesis: A mechanism for maternal tolerance of the fetus. Placenta. 2006; epub advance of print August 23: doi:10.1016/j.placenta.2006.07.003.
- Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci. 1996;93:705-708.
- Adams KA, Nelson JL. Microchimerism: An investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127-1131.
- Maloney S, Smith AG, Furst DE, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999,4:41-47.
- Nelson JL. Maternal-fetal immunology and autoimmune disease. Is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39:191-194.
- Nelson JL, Furst DE, Maloney S, et al. Microchimerism and HLA-compatible relationships of pregnancy in SSc. Lancet. 1998;351:559-562.
- Lambert NC, Erickson TD, Zhen Y, et al. Quantification of maternal microchimerism by HLA specific real-time PCR. Studies of healthy women and women with scleroderma. Arthritis Rheum. 2004;50:906-914.
- Loubiere LS, Lambert NC, Flinn L, et al. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages, and NK cells. Lab Invest. 2006; advance online September 11: doi:10.1038/labinvest. 3700471.
- Reed AM, Picornell YJ, Harwood A, Kredich DW. Chimerism in children with juvenile dermatomyositis. Lancet. 2000;356:2156-2157.
- Artlett CM, Ramos R, Jiminez SA, et al. Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Lancet. 2000;356:2155-2156.
- Reed AM, McNallan K, Wettstein P, Vehe R, Ober C. Does HLA-dependent chimerism underlie the pathogenesis of juvenile dermatomyositits? J Immunol. 2004;172:5041-5046.
- Stevens AM, Hermes H, Rutledge R, Buyon J, Nelson JL. Maternal microchimerism has myocardial tissue-specific phenotype in neonatal lupus congenital heart block. Lancet. 2003;362:1617-1623.
- Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75-80.
- Stevens AM, Mullarkey ME, Pang JM, et al. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab Invest. 2004;84:1603-1609.
- Portanova JP, Kotzin BL. Lupus-like autoimmunity in murine graft-versus-host disease. Concepts Immunopathol. 1988;6:119-140.
- Stevens AM, Tsao B, Hahn B, et al. Maternal HLA class II compatibility in men with systemic lupus erythematosus. Arthritis Rheum. 2005;52:2768-2773.
- Stevens AM. Microchimeric cells in systemic lupus erythematosus: Targets or innocent bystanders? Lupus. 2006;15:820-826
- Hovinga IC, Koopmans M, Baelde HJ, et al. Chimerism occurs twice as often in lupus nephritis as in normal kidneys. Arthritis Rheum. 2006;54:2944-2950.

