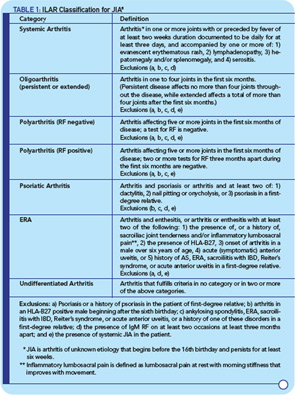
When the International League of Associations for Rheumatology (ILAR) proposed new classification criteria for juvenile arthritis in the 1990s, the term juvenile idiopathic arthritis (JIA) was introduced, and the category of enthesitis-related arthritis (ERA) was created to capture children with SpA. The architects of the JIA criteria emphasized the aim of defining relatively homogeneous and mutually exclusive categories based on predominant clinical and laboratory features. JIA criteria have since been revised to improve their applicability and reduce the number of children with arthritis who defy classification (see Table 1, p. 17).8
The ILAR criteria have been generally accepted and are used to define different phenotypes for genome-wide association studies aimed at discovering susceptible alleles.9 In addition, there is now evidence from peripheral blood–gene expression studies that the ILAR criteria identify biologically different groups of patients including ERA early in the disease course.10 However, it is also becoming evident that within JIA subtypes such as systemic and polyarthritis, there is heterogeneity that may portend different disease severity and outcome.11,12 Refining JIA classification with the use of genomic evidence in addition to clinical features will likely be a major focus of future studies and may enable better prediction of therapeutic response and outcome.
Despite overall improvement in the classification of juvenile arthritis using the ILAR criteria, the entity of pediatric SpA remains problematic from several perspectives. Many rheumatologists view SpA in children and adults on a continuum, with age of onset associated with phenotypic differences. However, the ERA category excludes patients with psoriasis or even a family history of psoriasis, and the presence of dactylitis or nail pitting in a child with arthritis places them in a separate psoriatic arthritis category even in the absence of psoriasis.8 Although this categorization may offer greater clarity in some situations, it can create confusion because it represents a distinct approach to the classification of SpA in children compared with adults.
In addition to common clinical features in the SpA group of diseases, there is also evidence for overlapping genetic predisposition and pathogenic mechanisms, most notably IL23R polymorphisms associated with susceptibility to psoriasis and AS, as well as inflammatory bowel disease (IBD) and activation of the Th17 axis.13-16 It seems likely that pediatric and adult-onset SpA will exhibit similar genetic and pathogenic overlaps. In addition to the issues raised above, the ILAR classification system does not explicitly deal with juvenile AS, reactive arthritis, or arthritis associated with IBD. Most children with juvenile AS (those who meet modified New York criteria by age 16 years) would be expected to meet criteria for ERA, although it is conceivable that some might not. More importantly, it makes little sense to communicate the concept of ERA to patients, parents, and other health care providers if the diagnosis is actually juvenile AS, because the term ERA conveys much less information about prognosis and long-term outcome.
The Relationship Between Inflammation and Bone Formation
Important advances in the treatment of AS have been made in the last decade, largely as a result of the use of TNF-a inhibitors that improve symptoms and signs of disease, including pain and inflammation.17 However, there is evidence that TNF-a blockade does not prevent aberrant bone formation in the axial skeleton.18,19 This finding is consistent with results from a mouse model of SpA, where the incidence and severity of spontaneous joint ankylosis were unaffected by TNF-a inhibition.20 Other studies demonstrate that excess TNF-a actually inhibits bone formation through effects on both the Wnt and BMP pathways, as well as by promoting osteoclast development and activation.21,22