Spondylarthritis (SpA) refers to a group of disorders with overlapping clinical features, estimated to affect between 0.5% and 2% of the population.1,2 Most often included in the SpA group are ankylosing spondylitis (AS), which constitutes about half of SpA, reactive arthritis, the arthritides associated with psoriasis and inflammatory bowel disease, and undifferentiated SpA. Although the majority of SpA starts in the third or fourth decade of life, a significant proportion of patients (10–20%) begin to experience symptoms during childhood. In pediatric rheumatology clinics, SpA accounts for up to 15–20% of the cases of inflammatory arthritis, with oligoarthritis, polyarthritis, and systemic juvenile arthritis accounting for the majority.
The most severe and often most debilitating form of SpA, AS, involves the axial skeleton and causes inflammation in the sacroiliac joints, vertebral bodies and intervertebral discs, and facet joints. Structural remodeling results in pathological bone formation in the form of osteophytes and syndesmophytes. These structures bridge vertebral bodies, leading to reduced spinal mobility and eventual fusion. At its earliest stages, SpA is often undifferentiated (i.e., lacks defining clinical or radiographic features) and may not involve the spine. The lack of axial involvement and related symptoms such as inflammatory back pain is particularly notable in children, who instead have a greater tendency to develop enthesitis and arthritis in the hips and other large joints of the lower extremities.3
Progression from undifferentiated SpA to AS is unpredictable in adults and children, and there is often a long lag period (8–11 years) between the onset of symptoms and a diagnosis of AS.4-6 Prompt recognition and management of early axial SpA, whether presenting in children or adults, is our best hope to alter the disease course and improve long-term outcomes. However, identifying those patients with undifferentiated SpA who are most likely to develop axial disease and AS, as well as appropriate pathways and targets for therapeutic intervention, remain important unmet needs in SpA.
The Classification of SpA
Recognizing SpA in children and distinguishing it from other forms of juvenile arthritis can be a challenge. Rosenberg and Petty recognized the need for specific classification criteria in the early 1980s and proposed the concept of the seronegative enthesopathy and arthropathy (SEA) syndrome. This syndrome has been defined as enthesitis with arthralgia or arthritis in children less than 17 years of age who also lack rheumatoid factor (RF) and antinuclear antibodies.7 The features of the SEA syndrome helped distinguish SpA from juvenile arthritis, classified at the time as juvenile rheumatoid or juvenile chronic arthritis, without relying on evidence of axial involvement.
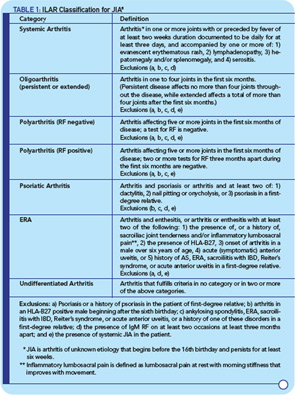
When the International League of Associations for Rheumatology (ILAR) proposed new classification criteria for juvenile arthritis in the 1990s, the term juvenile idiopathic arthritis (JIA) was introduced, and the category of enthesitis-related arthritis (ERA) was created to capture children with SpA. The architects of the JIA criteria emphasized the aim of defining relatively homogeneous and mutually exclusive categories based on predominant clinical and laboratory features. JIA criteria have since been revised to improve their applicability and reduce the number of children with arthritis who defy classification (see Table 1, p. 17).8
The ILAR criteria have been generally accepted and are used to define different phenotypes for genome-wide association studies aimed at discovering susceptible alleles.9 In addition, there is now evidence from peripheral blood–gene expression studies that the ILAR criteria identify biologically different groups of patients including ERA early in the disease course.10 However, it is also becoming evident that within JIA subtypes such as systemic and polyarthritis, there is heterogeneity that may portend different disease severity and outcome.11,12 Refining JIA classification with the use of genomic evidence in addition to clinical features will likely be a major focus of future studies and may enable better prediction of therapeutic response and outcome.
Despite overall improvement in the classification of juvenile arthritis using the ILAR criteria, the entity of pediatric SpA remains problematic from several perspectives. Many rheumatologists view SpA in children and adults on a continuum, with age of onset associated with phenotypic differences. However, the ERA category excludes patients with psoriasis or even a family history of psoriasis, and the presence of dactylitis or nail pitting in a child with arthritis places them in a separate psoriatic arthritis category even in the absence of psoriasis.8 Although this categorization may offer greater clarity in some situations, it can create confusion because it represents a distinct approach to the classification of SpA in children compared with adults.
In addition to common clinical features in the SpA group of diseases, there is also evidence for overlapping genetic predisposition and pathogenic mechanisms, most notably IL23R polymorphisms associated with susceptibility to psoriasis and AS, as well as inflammatory bowel disease (IBD) and activation of the Th17 axis.13-16 It seems likely that pediatric and adult-onset SpA will exhibit similar genetic and pathogenic overlaps. In addition to the issues raised above, the ILAR classification system does not explicitly deal with juvenile AS, reactive arthritis, or arthritis associated with IBD. Most children with juvenile AS (those who meet modified New York criteria by age 16 years) would be expected to meet criteria for ERA, although it is conceivable that some might not. More importantly, it makes little sense to communicate the concept of ERA to patients, parents, and other health care providers if the diagnosis is actually juvenile AS, because the term ERA conveys much less information about prognosis and long-term outcome.
The Relationship Between Inflammation and Bone Formation
Important advances in the treatment of AS have been made in the last decade, largely as a result of the use of TNF-a inhibitors that improve symptoms and signs of disease, including pain and inflammation.17 However, there is evidence that TNF-a blockade does not prevent aberrant bone formation in the axial skeleton.18,19 This finding is consistent with results from a mouse model of SpA, where the incidence and severity of spontaneous joint ankylosis were unaffected by TNF-a inhibition.20 Other studies demonstrate that excess TNF-a actually inhibits bone formation through effects on both the Wnt and BMP pathways, as well as by promoting osteoclast development and activation.21,22
Studies on the relationship between inflammation and syndesmophyte formation in AS patients suggest that these bony bridges tend to form at sites of vertebral inflammation, but most often after the inflammation has resolved.23–25 If these data are confirmed, they would suggest that although TNF-a–driven inflammation could be an important prerequisite to abnormal bone formation, the two processes may be mediated by different factors. If this is the case, TNF-a blockade very early in the course of disease might be beneficial, while later in disease it might permit new bone formation to proceed. There may be additional targets to inhibit pathological bone formation.
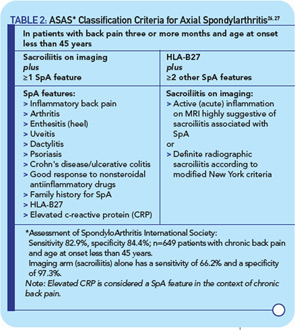
Identifying patients very early in the course of disease before the development of radiographic changes (and hence before they meet criteria for AS) has become a high priority in SpA research. The Assessment of SpondyloArthritis International Society (ASAS) workgroup recently reported criteria that identify patients with preradiographic axial SpA.26,27 This important advance was accomplished through a consensus approach using expert opinions and then validated in a multicenter study. The new criteria identify individuals with preradiographic axial SpA as well as those that meet the stricter modified New York criteria for AS and thus capture a large spectrum of axial disease (see Table 2, at right).
The new classification criteria incorporate evidence of active sacroiliitis on MRI or plain films, together with other clinical or laboratory features of SpA, most of which were included in the European Spondyarthropathy Study Group or Amor criteria that defined undifferentiated SpA. The axial SpA category includes individuals with psoriasis, dactylitis, and/or IBD. In 649 patients with chronic back pain who were younger than age 45 years, sensitivity and specificity were 83% and 84%, respectively. The ASAS group also refined clinical criteria for inflammatory back pain, which is now considered as chronic back pain (greater than or equal to three months in duration) of insidious onset, beginning at 40 years of age or younger, that improves with exercise but not with rest, and occurs at night with improvement on getting up. The new axial SpA criteria will enable clinical trials to address whether early aggressive treatment changes the course and outcome of disease, in particular the development and progression of bony changes such as osteophytes and syndesmophytes.
Treatment of SpA in Children
Current approaches to the treatment of SpA in children are based largely on clinical experience, because no controlled clinical trials have been published. Guidelines for adult patients with AS established by ASAS and modified by SPARTAN (an organization that fosters SpA research and education) for the U.S. population would be applicable to only the fraction of children who meet modified New York criteria.28 In patients with disease more broadly defined as pediatric SpA (such as ERA), nonsteroidal antiinflammatory drugs can help reduce inflammation and pain but are rarely adequate to control disease; there is no evidence that sulfasalazine or methotrexate are beneficial.29 Activity and physical therapy are widely recommended and considered to be beneficial, but more evidence is needed to better define the role of these modalities.
Given the efficacy of TNF-a inhibitors in adults with SpA, it is reasonable to predict that they will be beneficial in children. Indeed, small open-label studies of TNF-a inhibitors in pediatric patients with undifferentiated SpA have shown improvement in inflammation and the number of active joints and tender entheses.30,31 However, concern over the risk of lymphoma and other malignancies means that every decision to use these agents must be an informed one. Assessing the actual risk of TNF-a blockade in patients with rheumatic and other inflammatory diseases has been confounded by the concomitant use of other medications and lack of uniform data collection. Additional data from postmarketing surveillance and registries will be important to determine long-term safety of these and other biologics. For now, the decision to use TNF-a inhibitors involves weighing risks with potential benefits in the treatment of what can otherwise be a very debilitating disease with potentially irreversible sequelae.
Separate classification criteria for pediatric SpA have been developed to recognize signs and symptoms of SpA that are more common in children and reduce reliance on clinical features reflecting axial disease that are frequently lacking. Although this is important to capture the broad spectrum of undifferentiated SpA, it has become increasingly important to identify children with undifferentiated SpA who also have axial involvement. The ASAS criteria for pre-radiographic axial SpA provide a tangible starting point, although it may be possible to relax the requirement for three months of back pain, because this symptom is unusual in children.
Prospects for Genetics
The discovery of several novel genetic polymorphisms that, together with HLA-B27, explain a large proportion of predisposition to AS, provide a unique opportunity to determine the value of these markers to predict outcomes and identify children who may benefit from early aggressive therapy.14 Until pediatric SpA classification is formally re-addressed, a reasonable interim approach might be hierarchical:
- Identify children with juvenile AS (those under age 16 who meet the modified New York criteria);
- Apply the ASAS criteria to identify axial SpA when possible; and
- Use the ILAR system to identify undifferentiated SpA including both ERA and psoriatic arthritis.
Dr. Colbert is chief of the pediatric translational research branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health in Bethesda, Md.
References
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15-25.
- Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379-1390.
- Hofer M. Spondylarthropathies in children—are they different from those in adults? Best Pract Res Clin Rheumatol. 2006;20:315-328.
- Baraliakos X, Listin J, Brandt J, et al. Clinical response to discontinuation of anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther. 2005;7:R439-R444.
- Flatø B, Hoffmann-Vold AM, Reiff A, et al. Long-term outcome and prognostic factors in enthesitis-related arthritis: A case-control study. Arthritis Rheum. 2006;54:3573-3582.
- Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61-66.
- Rosenberg AM, Petty RE. A syndrome of seronegative enthesopathy and arthropathy in children. Arthritis Rheum. 1982; 25:1041-1047.
- Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J Rheumatol. 2004; 31:390-392.
- Duffy CM, Colbert RA, Laxer RM, Schanberg LE, Bowyer SL. Nomenclature and classification in chronic childhood arthritis: Time for a change? Arthritis Rheum. 2005;52:382-385.
- Barnes MG, Gromm AA, Thompson SD, et al. Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60, 2102-2112.
- Fall N, Barnes M, Thornton S, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793-3804.
- Griffin TA, Barnes MG, Ilowite NT, et al. Gene expression signatures in polyarticular juvenile idiopathic arthritis demonstrate disease heterogeneity and offer a molecular classification of disease subsets. Arthritis Rheum. 2009;60:2113-2123.
- Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80: 273-290.
- Wellcome Trust Case Control Consortium, Australo-Anglo-American Spondylitis Consortium (TASC), Burton PR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329-1337.
- Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463.
- Layh-Schmitt G, Colbert RA. The interleukin-23/interleukin-17 axis in spondyloarthritis. Curr Opin Rheumatol. 2008;20:392-397.
- LaSalle SP, Deodhar AA. Appropriate management of axial spondyloarthritis. Curr Rheumatol Rep. 2007;9:375-382.
- van der Heijde D, Landewé R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008; 58:3063-3070.
- van der Heijde D, Landewé R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58: 1324-1331.
- Lories RJ, Derese I, de Bari C, Luyten FP. Evidence for uncoupling of inflammation and joint remodeling in a mouse model of spondylarthritis. Arthritis Rheum. 2007; 56: 489-497.
- Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156-163.
- Yamazaki M, Fukushima H, Shin M, et al. TNFa represses BMP signaling by interfering with the DNA binding of smads through the activation of NF-kB. J Biol Chem. 2009;284: 35987-35995.
- Baraliakos X, Listing J, Rudwaleit M, Sieper J, Braun, J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther. 2008;10:R104.
- Appel H, Sieper J. Spondyloarthritis at the crossroads of imaging, pathology, and structural damage in the era of biologics. Curr Rheumatol Rep. 2008;10:356-363.
- Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: Evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;60:93-102.
- Rudwaleit M, Landewé R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68:770-776.
- Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann Rheum Dis. 2009;68:777-783.
- Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2006;65:442-452.
- Burgos-Vargas R. A case of childhood-onset ankylosing spondylitis: Diagnosis and treatment. Nat Clin Pract Rheumatol. 2009; 5:52-27.
- Henrickson M, Reiff A. Prolonged efficacy of etanercept in refractory enthesitis-related arthritis. J Rheumatol. 2004;31:2055-2061.
- Tse SM, Burgos-Vargas R, Laxer RM. Anti-tumor necrosis factor alpha blockade in the treatment of juvenile spondylarthropathy. Arthritis Rheum. 2005;52:2103-2108.