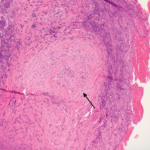
Based on the classification system developed by the Chapel Hill Consensus Conference, anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis is defined as a necrotizing vasculitis involving small vessels that is associated with myeloperoxidase (MPO) ANCA or proteinase 3 (PR3) ANCA and displays minimal immune deposits. The mechanism behind the pathogenesis of ANCA-associated vasculitis is not fully understood.
Granulomatosis with polyangiitis (GPA) is a subset of ANCA-associated vasculitis defined as a necrotizing granulomatous inflammation usually involving the respiratory tract and necrotizing vasculitis involving small and medium vessels, which is commonly associated with glomerulonephritis. Ocular vasculitis and pulmonary capillary inflammation with hemorrhage are also seen in GPA, and a limited form of GPA with ANCA positivity and manifestations only in the respiratory tract is also possible.1
GPA is more common in the Caucasian population with ancestry from northern Europe than in other populations and has a peak incidence between ages of 60 and 70 years. Numerous infectious, environmental and pharmacologic triggers for GPA exist, and the presentation of patients with GPA can vary a lot.2
Clinical manifestations of GPA vary widely and may include constitutional symptoms (fever, weight loss, myalgia, arthralgia), ocular involvement (scleritis, retinal vasculitis), ear, nose and throat involvement (sinusitis, nasal ulcers), respiratory tract involvement (cough, shortness of breath, alveolar hemorrhage, lung infiltrates, lung nodules, tracheal lesions), renal involvement (glomerulonephritis, proteinuria, hematuria, renal failure), nervous system involvement (headache, peripheral neuropathy, mononeuritis multiplex, cerebral vascular lesions, meningitis) or cutaneous lesions (purpura, subcutaneous nodules, ulcerations).3 Cardiac involvement, less frequent than other organ system involvement, can manifest as pericarditis, myocarditis, endocarditis or coronary vascular disease.4
The diagnosis of GPA is based on the patient’s clinical presentation and supporting laboratory and histologic findings. However, no well-established diagnostic rules or criteria exist. A positive ANCA is not required to diagnose GPA, and diseases that present similarly to a small vessel vasculitis must be excluded.
Infective endocarditis can present similarly to small vessel vasculitis. Clinical findings of fever, weight loss, myalgia and arthralgia, pulmonary infiltrates, glomerulonephritis and purpuric rash can be seen in both conditions. Many laboratory findings are seen in both conditions as well, such as elevated inflammatory markers, low complements and the presence of autoantibodies. In particular, ANCA, which is regarded as a very specific marker for vasculitis, can be positive in infective endocarditis as well.
In a recent prospective study of 109 patients with infective endocarditis, 18% had a positive ANCA on immunofluorescence testing (cytoplasmic [C-ANCA] or perinuclear [P-ANCA]) and 8% had a positive ANCA on enzyme-linked immunosorbent assay (ELISA) testing (PR3-ANCA or MPO-ANCA). In addition, 35% had a positive rheumatoid factor test, 16% had a positive anti-nuclear antibody (ANA) test and 23% was positive for anti-cardiolipin antibodies. Younger age, echocardiographic vegetations and elevated immunoglobulin G (IgG) levels were associated with a positive C- or P-ANCA.5