There is no doubt that the management of symptoms and pain associated with OA needs to be studied more thoroughly. Not only should we better understand the mechanisms of such symptoms but also, as recently proposed, we should “think outside the joint.”13 Recent findings indicate that neuropathic pain may contribute substantially to OA disease symptoms in a significant number of patients.14 The thinking surrounding the treatment of OA may be somewhat too focused on pain, a subjective symptom that may originate from many sources, particularly in elderly patients.
The ultimate vision for the treatment of OA has been to find agents that can reduce or stop the progression of the disease. Thus far, no such treatment has been approved by regulatory authorities as meeting the appropriate guidelines and criteria. However, a number of drugs and agents, including doxycycline, chondroitin sulfate, and glucosamine sulfate have been demonstrated by X-ray to effectively reduce joint space narrowing in patients with knee OA; of note, licofelone has been shown by magnetic resonance imaging (MRI) to decrease cartilage volume loss.15-20 Moreover, diacerein was shown to reduce the progression of hip OA.21 However, disagreement over the interpretation of the results of some of these studies is notable and pertains to issues regarding sample size, sensitivity and reliability of imaging technology used, and the patient population studied.22,23
DMOAD Development Is a Risky Business for Many
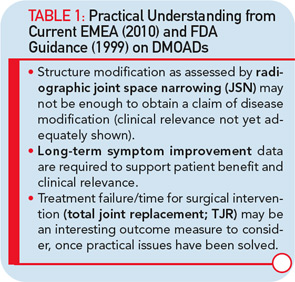
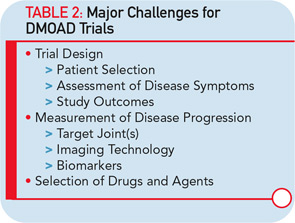
Major hurdles are responsible for the limited effort to develop new disease-modifying OA drugs (DMOADs). These hurdles can be explained quite simply by two major factors that are intertwined. The first is the key basis for the development of DMOADs, which is financial support from industry. Second is the regulatory environment that provides guidelines for the development of DMOADs.24,25 The research and development programs for DMOADs are very costly; to many investigators in the field, the regulations seem outdated, making it a high-risk investment for the industry. The perception of the current regulatory guidelines on DMOAD development is that they do not provide updated and optimal guidance and, very importantly, that key elements of these guidelines do not incorporate recent advances in the field of OA research. These advances could significantly impact study design and conduct (see Table 1). We can only hope that recent initiatives to open the dialogue for discussion with regulatory agencies will be successful at resolving these issues.