Experience with chemical biomarkers in DMOAD trials is very limited; therefore, it is premature at this time to conclude on their usefulness in such studies.30
Conclusions
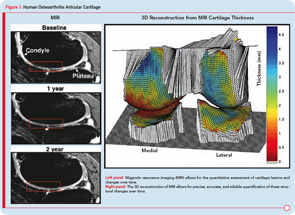
Research into the development of new and innovative DMOADs must continue, even if this effort has not been completely successful the last few decades, because there is a desperate need for effective and safe DMOADs. Moreover, the knowledge gathered over the past twenty or thirty years should not be underestimated, but rather should guide DMOAD development programs toward new heights and promising therapeutic targets (see Figure 1). However, any DMOAD program will remain in the dark until improved and comprehensive guidelines become available. In the meantime, we can only hope that the dream of a safe and effective DMOAD becomes a reality sooner rather than later.
Disclosure and Acknowledgments
Drs. Pelletier and Martel-Pelletier are consultants for and shareholders in ArthroLab Inc. and ArthroVision Inc., as well as consultants for AstraZeneca, Bioiberica, Boehringer Ingelheim, CEVA, Regeneron Pharmaceuticals, Rottapharm, Servier, TRB Chemedica, Virbac and Winston Laboratories.
The authors wish to thank Santa Fiori for her assistance with the preparation of this paper.
Drs. Pelletier and Martel-Pelletier are both professors of medicine and chairholders of the chair in osteoarthritis at the University of Montreal, and directors of the Osteoarthritis Research Unit at the Notre-Dame Hospital in Montreal, Quebec, Canada.
References
- Uhlig T, Slatkowsky-Christense B, Moe RH, Kvien TK. The burden of osteoarthritis: The societal and the patient perspective. Therapy. 2010;7:605-619.
- Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499.
- Martel-Pelletier J, Lajeunesse D, Fahmi H, Tardif G, Pelletier JP. New thoughts on the pathophysiology of osteoarthritis: One more step toward new therapeutic targets. Curr Rheumatol Rep. 2006;8:30-36.
- Martel-Pelletier J, Pelletier JP. New insights into the major pathophyiological processes responsible for the development of osteoarthritis. Semin Arthritis Rheum. 2005;34:6-8.
- Berthiaume MJ, Raynauld JP, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with the progression of knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556-563.
- Ding C, Martel-Pelletier J, Pelletier JP, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: A cross-sectional study. J Rheumatol. 2007;34:776-784.
- Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis. 2008;67:683-688.
- Pelletier JP, Raynauld JP, Abram F, Haraoui B, Choquette D, Martel-Pelletier J. A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage. 2009;17:822-824.
- Pelletier JP, Boileau C, Altman RD, Martel-Pelletier J. Animal models of osteoarthritis. In: Rheumatology.vol. 2, 5th edn. Hochberg M, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Philadelphia, PA: Elsevier; 2010:1731-1739.
- d’Anjou MA, Troncy E, Moreau M, et al. Temporal assessment of bone marrow lesions on magnetic resonance imaging in a canine model of knee osteoarthritis: Impact of sequence selection. Osteoarthritis Cartilage. 2008;16:1307-1311.
- Herrero-Beaumont G, Roman-Blas JA, Castaneda S, Jimenez SA. Primary osteoarthritis no longer primary: Three subsets with distinct etiological, clinical, and therapeutic characteristics. Semin Arthritis Rheum 2009;39:71-80.
- FDA Joint Meeting of the Drug Safety and Risk Management Advisory Committee with the Anesthetic and Life Support Drugs Advisory Committee and Nonprescription Drugs Advisory Committee. June 23–29, 2009. In Adelphi, Maryland.
- Clauw DJ, Witter J. Pain and rheumatology: Thinking outside the joint. Arthritis Rheum. 2009;60:321-324.
- Imamura M, Imamura ST, Kaziyama HH, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: A controlled analysis. Arthritis Rheum. 2008;59:1424-1431.
- Brandt KD, Mazzuca SA, Katz BP, et al. Effects of doxycycline on progression of osteoarthritis: Results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52:2015-2025.
- Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: The study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
- Michel BA, Stucki G, Frey D, De Vathaire F, Vignon E, Bruehlmann P, Uebelhart D. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: A randomized, controlled trial. Arthritis Rheum. 2005;52:779-786.
- Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet. 2001;357:251-256.
- Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: A 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113-2123.
- Raynauld JP, Martel-Pelletier J, Bias P, et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: A first multicentre clinical trial using quantitative MRI. Ann Rheum Dis. 2009;68:938-947.
- Dougados M, Nguyen M, Berdah L, Mazieres B, Vignon E, Lequesne M. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip. Arthritis Rheum. 2001;44:2539-2547.
- Buckland-Wright C. Review of the anatomical and radiological differences between fluoroscopic and non-fluoroscopic positioning of osteoarthritic knees. Osteoarthritis Cartilage. 2006;14:A19-A31.
- Brandt KD, Mazzuca SA. Lessons learned from nine clinical trials of disease-modifying osteoarthritis drugs. Arthritis Rheum. 2005;52:3349-3359.
- FDA. Clinical development programs for drugs, devices and biological products intended for the treatment of OA. Published July 1999. Available at www.fda.gov/Cber/gdins/osleo.htm.
- Committee for Medicinal Products for Human Use (CHMP). Guideline on Clinical Investigation of Medicinal Products Used in the Treatment of Osteoarthritis (CPMP/EWP/784/97 Rev 1). London: European Medicines Agency, January 2010.
- Pelletier JP, Raynauld JP, Martel-Pelletier J. Designing an OA clinical trial. Osteoarthritis Cartilage. 2009;17:S6.
- Altman RD, Abadie E, Avouac B, et al. Total joint replacement of hip or knee as an outcome measure for structure modifying trials in osteoarthritis. Osteoarthritis Cartilage. 2005;13:13-19.
- Martel-Pelletier J, Pelletier J. Quantitative MRI: A novel assessment technology for the measurement of knee osteoarthritis structural changes. European Musculoskeletal Review. 2009;4:58-59.
- Raynauld JP, Martel-Pelletier J, Beaulieu A, et al. An open-label pilot study evaluating by magnetic resonance imaging the potential for a disease-modifying effect of celecoxib compared to a modelized historical control cohort in the treatment of knee osteoarthritis. Semin Arthritis Rheum, 2010;40:185-192.
- Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:346-356.