With the aging of the world’s population, the burden of osteoarthritis (OA) is rising relentlessly, posing enormous costs and challenges to the healthcare system. OA is by far the most common musculoskeletal condition and a major source of chronic pain and disability.1 The increasing number of patients seeking treatment for OA could very well cause a global financial crisis in the near future. Any hope for avoiding being hit by the storm is fading because few advances in the development of treatments to reduce the progression of the disease have been achieved.
Although some progress has occurred in the symptomatic treatment of the disease, it has been relatively modest, leaving a significant number of patients to live with a painful and crippling disease. Surgery, particularly of weight-bearing joints, has been a useful treatment for end-stage OA.2 The need for such an approach is the obvious result of the lack of an otherwise effective medical treatment. The questions to ask at this time are important: What went wrong with the development of therapy in this field of medicine? Why are we faced with such a desperate situation after so much time and effort? Going back in time may help us to understand the situation we are facing today concerning the management and treatment of OA.
Historical Perspective
Decades ago, the burden of OA was much less of a concern from a medical point of view due to the shorter life expectancy of the population. Significant progress in medical care and, more particularly, in the treatment of medical conditions such as cardiovascular diseases and cancer has contributed substantially to increasing life expectancy as well as increasing awareness of the importance of the quality of life. Degenerative diseases linked to aging, such as OA and Alzheimer’s disease, are the aftermath of such a demographic change. This unforeseen increase in the number of patients suffering from these chronic debilitating diseases has resulted in a shortage of effective solutions.
Despite many experimental obstacles, important progress has been made in understanding the pathophysiology of OA. Different biomechanical and biochemical pathophysiological pathways leading to disease development and progression have been identified through major research efforts.3.4 Moreover, clinical and epidemiological studies and new imaging technology have helped identify important risk factors associated with OA progression and symptoms. For instance, a number of risk factors for disease progression, such as bone marrow lesions, meniscal alterations, and synovitis, have also been associated with OA symptoms.5-8 Interestingly, these latter findings are consistent with those from preclinical research in large animal models, providing support to the results of such investigation.9,10
The increasing number of patients seeking treatment for OA could very well cause a global financial crisis in the near future. Any hope for avoiding being hit by the storm is fading because few advances in the development of treatments to reduce the progression of the disease have been achieved.
An important underlying question in this field relates to the etiopathogenesis of the disease and whether OA is homogeneous in nature. Is the process leading to OA the same from one joint to another, from weight-bearing to non–weight bearing joints, from peripheral to central joints, and between individuals? This query remains largely unanswered and is most relevant in the context of the development of disease-modifying treatments for OA.11 Moreover, the fact that a considerable amount of information available on OA is derived from studies performed on weight-bearing joints may possibly create a bias in the development of new therapies. In that context, the choice of preclinical animal models to study OA should also be carefully examined, ensuring the reproducibility of disease characteristics as in the natural disease.9
OA Treatment: From Symptoms to Structural Changes
The knowledge gained from preclinical and clinical research in OA has led to the development of a large number of programs aimed at targeting different disease mechanisms responsible for disease symptoms and structural changes. As mentioned with regard to disease symptoms, a number of local and systemic treatments have been developed that can provide patients with some degree of relief, improving the quality of life.1,2 However, there remains a high level of unmet needs.1 Furthermore, some of the treatments, such as nonsteroidal antiinflammatory drugs and narcotics, can cause significant side effects, which are a concern in the aging OA population. Likewise, acetaminophen, the first-line agent recommended by most practice guidelines, has also been demonstrated to have potential toxicity; authorities now recommend its use with caution.12 The use of symptomatic slow-acting drugs in OA (SySADOA) has become an interesting alternative and has been proven effective, despite criticism.2
There is no doubt that the management of symptoms and pain associated with OA needs to be studied more thoroughly. Not only should we better understand the mechanisms of such symptoms but also, as recently proposed, we should “think outside the joint.”13 Recent findings indicate that neuropathic pain may contribute substantially to OA disease symptoms in a significant number of patients.14 The thinking surrounding the treatment of OA may be somewhat too focused on pain, a subjective symptom that may originate from many sources, particularly in elderly patients.
The ultimate vision for the treatment of OA has been to find agents that can reduce or stop the progression of the disease. Thus far, no such treatment has been approved by regulatory authorities as meeting the appropriate guidelines and criteria. However, a number of drugs and agents, including doxycycline, chondroitin sulfate, and glucosamine sulfate have been demonstrated by X-ray to effectively reduce joint space narrowing in patients with knee OA; of note, licofelone has been shown by magnetic resonance imaging (MRI) to decrease cartilage volume loss.15-20 Moreover, diacerein was shown to reduce the progression of hip OA.21 However, disagreement over the interpretation of the results of some of these studies is notable and pertains to issues regarding sample size, sensitivity and reliability of imaging technology used, and the patient population studied.22,23
DMOAD Development Is a Risky Business for Many
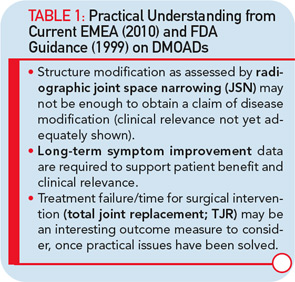
Major hurdles are responsible for the limited effort to develop new disease-modifying OA drugs (DMOADs). These hurdles can be explained quite simply by two major factors that are intertwined. The first is the key basis for the development of DMOADs, which is financial support from industry. Second is the regulatory environment that provides guidelines for the development of DMOADs.24,25 The research and development programs for DMOADs are very costly; to many investigators in the field, the regulations seem outdated, making it a high-risk investment for the industry. The perception of the current regulatory guidelines on DMOAD development is that they do not provide updated and optimal guidance and, very importantly, that key elements of these guidelines do not incorporate recent advances in the field of OA research. These advances could significantly impact study design and conduct (see Table 1). We can only hope that recent initiatives to open the dialogue for discussion with regulatory agencies will be successful at resolving these issues.
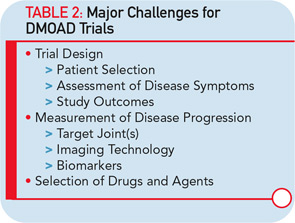
Based on experience from the conduct of DMOAD trials in the last decades, such studies present major challenges (see Table 2). A number of lessons have been learned and are worth sharing.23,26 First, protocols for these trials were highly variable from one study to another. For example, inclusion/exclusion criteria regarding disease severity, demographic, risk factors, and structural changes differed quite significantly among the studies and could have an obvious impact on the results, complicating the analyses and comparisons of the results of a particular drug with others. Efforts should be made to better standardize these criteria to ensure a homogeneous and representative population. This would also allow better standardization and balancing of the risks factors associated with the disease progression, which is essential in such studies.
The whole issue about the extent of effect that a DMOAD should have on disease symptoms to be acceptable is also problematic. To start with, the questionnaires and evaluation scales recommended and used in these studies have been imported from short-term trials dealing with the efficacy of OA treatment on symptoms. Are they reliable for long-term DMOAD studies? Maybe. Maybe not. This question is of the utmost importance and needs to be answered.
One of the real challenges in the development of DMOADs is finding an ideal study outcome measure. Ideally, a hard target such as the reduction in the need for total joint replacement is most interesting (see Table 1). However, as a practical matter, since the incidence of total joint replacement during clinical trials is fairly low, particularly in knee OA trials, the concept of a responder criteria combining the impact of DMOAD treatment of disease symptoms and progression of structural changes, has recently been advocated, and should be considered more carefully.27
The major goal of a DMOAD treatment should be to reduce the progression of joint structural changes, namely cartilage loss. There is a general consensus that a study duration of two years is realistic. The study joint should generally be a weight-bearing one (e.g., knee or hip), unless a specific indication for another joint (e.g., hand) is made. An important issue is whether studying one target joint only is adequate and representative of a drug’s effect. If the effect of treatment is systemic, should more than one joint be studied? This question is of high relevance and needs to be addressed.
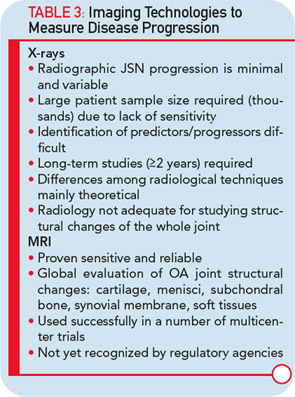
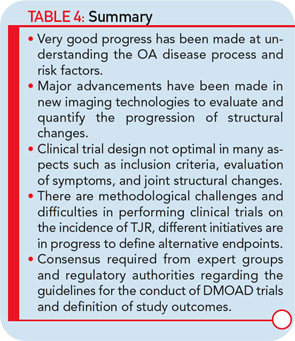
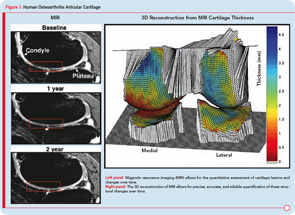
The imaging method used for the assessment of the DMOAD effect has traditionally been X-rays, which are still recommended today. Should this technology still be the gold standard, given the progress made in the field of OA imaging in the last decade? Probably not. This conclusion appears especially true when one realizes the limitations imposed by the use of X-rays (see Table 3).22,23 Advanced technology, such as MRI, can provide more comprehensive and reliable information on the progression of the disease and the effects of DMOAD treatment (see Table 3 and Table 4).20,28,29 MRI is an approved imaging modality and has been used as reference in many fields of research, including trials in cancer and central nervous system disease for a long time. The time has come for the field of OA research to follow suit because this technology can assist in the development of proof-of-concept DMOAD studies and reduce the number of patients to be included in phase II and III trials. Such a change will make DMOAD development programs more affordable and, at the same time, bring new hope to OA patients.
Experience with chemical biomarkers in DMOAD trials is very limited; therefore, it is premature at this time to conclude on their usefulness in such studies.30
Conclusions
Research into the development of new and innovative DMOADs must continue, even if this effort has not been completely successful the last few decades, because there is a desperate need for effective and safe DMOADs. Moreover, the knowledge gathered over the past twenty or thirty years should not be underestimated, but rather should guide DMOAD development programs toward new heights and promising therapeutic targets (see Figure 1). However, any DMOAD program will remain in the dark until improved and comprehensive guidelines become available. In the meantime, we can only hope that the dream of a safe and effective DMOAD becomes a reality sooner rather than later.
Disclosure and Acknowledgments
Drs. Pelletier and Martel-Pelletier are consultants for and shareholders in ArthroLab Inc. and ArthroVision Inc., as well as consultants for AstraZeneca, Bioiberica, Boehringer Ingelheim, CEVA, Regeneron Pharmaceuticals, Rottapharm, Servier, TRB Chemedica, Virbac and Winston Laboratories.
The authors wish to thank Santa Fiori for her assistance with the preparation of this paper.
Drs. Pelletier and Martel-Pelletier are both professors of medicine and chairholders of the chair in osteoarthritis at the University of Montreal, and directors of the Osteoarthritis Research Unit at the Notre-Dame Hospital in Montreal, Quebec, Canada.
References
- Uhlig T, Slatkowsky-Christense B, Moe RH, Kvien TK. The burden of osteoarthritis: The societal and the patient perspective. Therapy. 2010;7:605-619.
- Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499.
- Martel-Pelletier J, Lajeunesse D, Fahmi H, Tardif G, Pelletier JP. New thoughts on the pathophysiology of osteoarthritis: One more step toward new therapeutic targets. Curr Rheumatol Rep. 2006;8:30-36.
- Martel-Pelletier J, Pelletier JP. New insights into the major pathophyiological processes responsible for the development of osteoarthritis. Semin Arthritis Rheum. 2005;34:6-8.
- Berthiaume MJ, Raynauld JP, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with the progression of knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556-563.
- Ding C, Martel-Pelletier J, Pelletier JP, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: A cross-sectional study. J Rheumatol. 2007;34:776-784.
- Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis. 2008;67:683-688.
- Pelletier JP, Raynauld JP, Abram F, Haraoui B, Choquette D, Martel-Pelletier J. A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage. 2009;17:822-824.
- Pelletier JP, Boileau C, Altman RD, Martel-Pelletier J. Animal models of osteoarthritis. In: Rheumatology.vol. 2, 5th edn. Hochberg M, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Philadelphia, PA: Elsevier; 2010:1731-1739.
- d’Anjou MA, Troncy E, Moreau M, et al. Temporal assessment of bone marrow lesions on magnetic resonance imaging in a canine model of knee osteoarthritis: Impact of sequence selection. Osteoarthritis Cartilage. 2008;16:1307-1311.
- Herrero-Beaumont G, Roman-Blas JA, Castaneda S, Jimenez SA. Primary osteoarthritis no longer primary: Three subsets with distinct etiological, clinical, and therapeutic characteristics. Semin Arthritis Rheum 2009;39:71-80.
- FDA Joint Meeting of the Drug Safety and Risk Management Advisory Committee with the Anesthetic and Life Support Drugs Advisory Committee and Nonprescription Drugs Advisory Committee. June 23–29, 2009. In Adelphi, Maryland.
- Clauw DJ, Witter J. Pain and rheumatology: Thinking outside the joint. Arthritis Rheum. 2009;60:321-324.
- Imamura M, Imamura ST, Kaziyama HH, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: A controlled analysis. Arthritis Rheum. 2008;59:1424-1431.
- Brandt KD, Mazzuca SA, Katz BP, et al. Effects of doxycycline on progression of osteoarthritis: Results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52:2015-2025.
- Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: The study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
- Michel BA, Stucki G, Frey D, De Vathaire F, Vignon E, Bruehlmann P, Uebelhart D. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: A randomized, controlled trial. Arthritis Rheum. 2005;52:779-786.
- Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet. 2001;357:251-256.
- Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: A 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113-2123.
- Raynauld JP, Martel-Pelletier J, Bias P, et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: A first multicentre clinical trial using quantitative MRI. Ann Rheum Dis. 2009;68:938-947.
- Dougados M, Nguyen M, Berdah L, Mazieres B, Vignon E, Lequesne M. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip. Arthritis Rheum. 2001;44:2539-2547.
- Buckland-Wright C. Review of the anatomical and radiological differences between fluoroscopic and non-fluoroscopic positioning of osteoarthritic knees. Osteoarthritis Cartilage. 2006;14:A19-A31.
- Brandt KD, Mazzuca SA. Lessons learned from nine clinical trials of disease-modifying osteoarthritis drugs. Arthritis Rheum. 2005;52:3349-3359.
- FDA. Clinical development programs for drugs, devices and biological products intended for the treatment of OA. Published July 1999. Available at www.fda.gov/Cber/gdins/osleo.htm.
- Committee for Medicinal Products for Human Use (CHMP). Guideline on Clinical Investigation of Medicinal Products Used in the Treatment of Osteoarthritis (CPMP/EWP/784/97 Rev 1). London: European Medicines Agency, January 2010.
- Pelletier JP, Raynauld JP, Martel-Pelletier J. Designing an OA clinical trial. Osteoarthritis Cartilage. 2009;17:S6.
- Altman RD, Abadie E, Avouac B, et al. Total joint replacement of hip or knee as an outcome measure for structure modifying trials in osteoarthritis. Osteoarthritis Cartilage. 2005;13:13-19.
- Martel-Pelletier J, Pelletier J. Quantitative MRI: A novel assessment technology for the measurement of knee osteoarthritis structural changes. European Musculoskeletal Review. 2009;4:58-59.
- Raynauld JP, Martel-Pelletier J, Beaulieu A, et al. An open-label pilot study evaluating by magnetic resonance imaging the potential for a disease-modifying effect of celecoxib compared to a modelized historical control cohort in the treatment of knee osteoarthritis. Semin Arthritis Rheum, 2010;40:185-192.
- Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:346-356.