Despite the complex names and the different permutations of their component molecules, inflammasome structures share the common property of caspase activation on assembly. The NLRP component is thought to act as a sensor, either of exogenous or endogenous danger signals that are generated by infection and cell stress, to initiate the assembly of the complex, eventually leading to dimerization and activation of the caspases. The NLRPs are part of the NLR (NOD-like receptor) family of proteins that has many members and shares a similar domain structure. Most NLRs are composed of a leucine-rich repeat (LRR) domain, an oligomerization domain, and an effector region that mediates interactions with effector caspases through homotypic interactions that were first identified in apoptosis related caspases (see Figure 1, p. 19). Three families have been identified based on sequence homology and are named the NLRP (or NALP), IPAF, and NOD families (see Figure 2, p. 19).
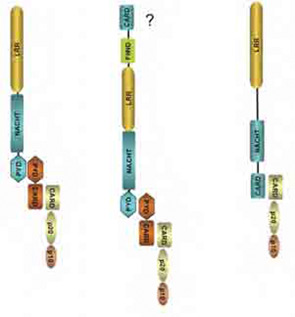
The NLRP1 inflammasome has a CARD domain at its C-terminus and may be able to associate directly with caspases. The NLRP3 inflammasome is composed of ASC and caspase-1, and the IPAF inflammasome does not contain ASC.
How sensing is performed by NLRPs is still poorly understood, but the LRR domain resembles that found in toll-like receptors, which have been shown to have a sensor function for ligands such as lipopolysaccharide. To date, three inflammasomes have been identified: NLRP1, NLRP3, and IPAF. ASC, a component of the NLRP1 and NLRP3 inflammasomes, acts as an adaptor to link the sensor to the effector caspase and is essential for its function. Absence of ASC blocks IL-1b and IL-18 processing. The final component is the pro-inflammatory caspase, which in man could either be caspase-1, -4, -5, or -12. Caspase-1 was previously also known as IL-1 converting enzyme, and its importance in processing IL-1b was described nearly 20 years ago.5,6 The properties of the other caspases have been studied in less detail. These components of the inflammasome are found in the cytoplasm, but there is variation in their tissue distribution, particularly of the NLRs. Immunohistochemistry showed that leucocytes express high levels of NLRP1 and 3, and epithelial cells differentially express these two proteins depending on the tissue.7
The most interesting part of the inflammasome story concerns the signals that lead to its activation. As previously mentioned, the NLR is thought to act as the sensor of endogenous or exogenous danger signals. This sensor function has been conserved throughout evolution, as the plant homologs of NLR, the resistance (R) genes, are critical for the plants’ defense against bacteria, fungi, and viruses. In humans, the list of inflammasome activators is ever expanding and includes components of infectious agents (e.g., Leishmania, Yersinia, Staphylococcus), particles (e.g., microcrystals), metabolic alterations (such as hyperglycemia and adenosine triphosphate concentration), and chemicals. In terms of human health and disease, inflammasome activation plays a key role in cryopyrin-associated periodic syndrome (CAPS), acute gouty inflammation, and the mechanism of action of vaccine adjuvants.
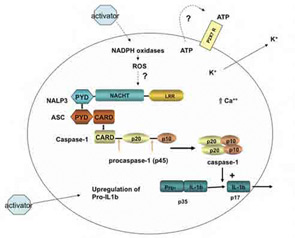
The macromolecular complex (inflammasome) consists of NLRP3, ASC, and procaspase-1. Assembly leads to activation of caspase-1 that in turn cleaves pro–IL-1b to produce biologically active IL-1b. The suggested scheme is based on available data for monosodium urate (MSU)–induced activation of macrophages. Cell binding or uptake of the activator leads to the generation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate hydrogen oxidases. This event activates the NLRP3 inflammasome. MSU crystals may also induce the secretion of ATP that in turn activates P2X7R. On activation of the P2X7 receptor, there is rapid exit of intracellular potassium that triggers the NLRP3 inflammasome. A rise in intracellular calcium is also required for the secretion of processed IL-1b.
Interest in the inflammasome was ignited by the identification of mutations in the NLRP3 gene as the molecular basis of CAPS. Patients with this condition had defied diagnosis for more than two decades until the identification of the genetic mutation and subsequent studies linking dysregulated IL-1b production by constitutive activity of the NLRP3 inflammasome.8-9 As IL-1 inhibitors (e.g., anakinra or IL-1Ra) were readily available, a proof-of-concept study could be rapidly performed, with the striking results we now know.10 These elegant translational studies closed the loop of pathogenesis and established the first link between inflammasome activation and disease in humans and rekindled our interest in the role of IL-1 in human disease.
