Currently, controlled trials in gout with rilonacept and canankinumab are in progress, and positive results have been reported as abstracts.24,25 It is obviously inappropriate to treat all cases acute gout in this way, but these agents promise to be an alternative therapy in patients who cannot tolerate nonsteroidal antiinflammatory drugs or colchicine. Other conditions have been successfully treated with anakinra, suggesting that inflammasome-mediated IL-1 production has a role in pathophysiology. These conditions include Still’s disease (both juvenile onset and adult types), Schnitzler’s syndrome (a condition of uriticaria, fever, bone pain, arthritis, and an immunoglobin M gammopathy), and acute arthritis due to calcium pyrophosphate dihydrate (CPPD). In all these cases, conventional treatment with antiinflammatories, steroids, and immunosuppressive drugs had not provided long-lasting relief. The successful results with IL-1Ra, sometimes used over prolonged periods, have been invaluable for patients with these conditions. Other indications for IL-1 blockade that may come into clinical reality are based on data from experimental models or early-phase clinical studies; these include diabetes mellitus, the prevention of asbestosis, and in the treatment of certain skin conditions. The concerns about therapy targeting IL-1, apart from efficacy and costs, are the risk for infections and long-term immunosuppression. However, the current data from clinical studies with IL-1 inhibitors have not signaled any major safety concern. The future of anti-IL-1–based treatments looks bright.
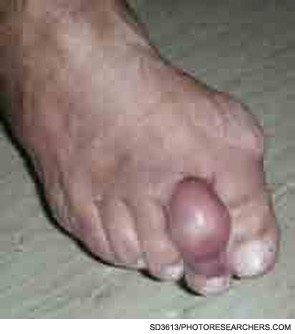
In the next few years, we can expect that the field of inflammasome research will continue to expand. Importantly, future studies will determine how these sensors of danger signals may be subverted to maintain chronic inflammation. As inflammation is a process common to many different diseases, from atherosclerosis to Parkinson’s disease, it is obviously important to understand how this process is maintained and how its modulation may change disease course.
Dr. So is professor of rheumatology and Dr. Pazár is in the service of rheumatology, both at the University Hospital Lausanne in Switzerland.
References
- Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease (*). Annu Rev Immunol. 2009;27:621-668.
- Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-426.
- Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119-21122.
- Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229-265.
- Kostura MJ, Tocci MJ, Limjuco G, et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989; 86:5227-5231.
- Thornberry NA, Bull HG, Calaycay JR, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768-774.
- Kummer JA, Broekhuizen R, Everett H, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443-452.
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301-305.
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1 beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319-325.
- Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607-612.
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237-241.
- So A, Desmedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28.
- Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517-1526.
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136-140.
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674-677.
- Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17-21.
- Kool M, Petrilli V, De Smedt T, et al. Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181: 3755-3759.
- Sharp FA, Ruane D, Claass B, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:870-875.
- Iyer SS, Pulskens, WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388-20393.
- Watanabe H, Gaide O, Petrilli V, et al. Activation of the IL-1 beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956-1963.
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007; 14:1583-1589.
- Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581-592.
- Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416-2425.
- So A, De Meulemeester M, Pikhlak A, et al. Canakinumab (ACZ885) vs. triamcinolone acetonide for treatment of acute flares and prevention of recurrent flares in gouty arthritis patients refractory to or contraindicated to NSAIDs and/or colchicine. Presented at the 75th Annual Scientific Meeting of the ACR; October 2009; Philadelphia.
- Terkeltaub R, Sundy J, Schumacher HR, et al. The IL-1 inhibitor rilonacept in treatment of chronic gouty arthritis: Results of a placebo-controlled, cross-over pilot study. Ann Rheum Dis. 2009;68:1613-1617.
