PMN, aggregating to neutrophilic microabscesses (see the left panel of Figure 2, p. 41)—frequently in the center of epitheloid cell granulomas—undergo cell death, eventually forming areas of geographic necrosis.20 This cell death may occur via NETosis, a cell death program that could play a central role in the induction of autoimmunity.22
Within the inflammatory lesions of granulomatous WG, lymphocytes together with follicular dendritic cells form germinal center-like structures (see the right panel of Figure 2, p. 41), a process very similar to rheumatoid synovium. These follicular structures provide a microenvironment for the development of autoreactive B cells (see below), which may link both hallmarks of WG, the granulomatous lesion as a site of autoantibody production and the autoimmune vasculitis. Surprisingly, another pathogenic process takes place in the granulomatous lesion (i.e., cartilage) (see the right panel of Figure 1, p. 41) and bone destruction (see the right panel of Figure 2, p. 41). This process has marked similarity to events in the rheumatoid synovium.
Autoantigens and Autoantibodies in the Granulomatous Lesion
Autoantibodies to constituents of PMN (e.g., PR3 [PR3-ANCA] or myeloperoxidase [MPO] [MPO-ANCA]) are the serological hallmarks of systemic vasculitis, and PR3-ANCA is strongly associated with WG. PR3 and MPO are abundant in neutrophil extracellular traps (NETs) formed by PMN that undergo nuclear disintegration and decondensation. NETs are web-like structures composed of chromatin bound to positively charged molecules (histones, neutrophil serine proteases). NETs play an important role physiologically and act in antimicrobial defense carried out by PMN.23 NETs have been found in glomerulonephritis of WG both in situ and in the bloodstream.24 Excess formation of NETs and their presentation of a modified PR3 also suggest a way by which dying PMN present in microabscesses and contribute to the necrotic areas in the granulomatous lesion and a subsequent rise of PR3-ANCA.20,25 In addition, a NETs-mediated release of factors such as B-lymphocyte stimulator by PMN could, for instance, promote the activation of B cells.26
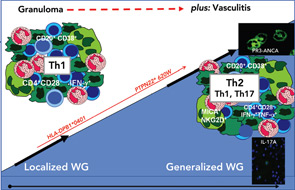
We have reported that germinal center-like structures containing T and B cells are often found within granulomatous lesions and hypothesized that germinal center-like structures might be an important element of a microenvironment that supports autoantibody-producing plasma cells (see references 21, 27, and 28 for a review of this). Indeed, we showed an accumulation of heavy chain variable region immunglobulin gene mutations in the endonasal mucosa of WG, pointing towards an influence of local autoantigens, such as PR3 on the generation of autoimmunity.29
