Systemic Sclerosis (SSc) is a complex systemic illness that is characterized by a heterogeneous mix of serious and sometimes life-threatening clinical manifestations. Because of the extensive involvement of internal organs (e.g., lung, heart, kidney, gastrointestinal system), patient care requires a comprehensive approach and a solid preparation to manage the clinical picture. Of manifestations in SSc, cardiac involvement is often clinically silent or occult and, indeed, may be missed without special study. The main signs and symptoms, however, are usually related to cardiomyopathy, pericarditis, conduction and rhythm disturbances, or vascular disorders.1 The impact of the cardiac disease on morbidity and mortality in SSc makes understanding of this manifestation a top priority for the rheumatologist caring for patients with this disease.
In the clinic, the evaluation of heart involvement in SSc can be difficult, since a variable pattern of symptoms like dyspnoea upon exertion, palpitations, dizziness, atypical chest pain, and syncope may occur. These features may overlap with other problems in SSc (e.g., lung disease) creating a dangerous “puzzle” associated with increased mortality—one that may challenge the physician’s skills. In addition to primary heart involvement, pulmonary hypertension and renal involvement can induce a secondary cardiac disease and dysfunction that are a harbinger of poor prognosis. By diagnosing heart involvement in SSc early in its course, timely and effective treatment measures can be initiated, optimizing the clinical outcome. Here we focus on the diagnosis of the different “puzzle” components of heart involvement in SSc as well as the available treatment.
Electric Piece of the Puzzle
Conduction defects and arrhythmias occur frequently in SSc as a result of fibrosis or ischemia of the conduction system. Arrhythmias may be a manifestation of early cardiac involvement and may be detected during ambulatory monitoring.2 Palpitations or syncope may occur, but usually patients are asymptomatic. Ventricular arrhythmias and sudden cardiac death are the most serious and dreaded complications. Pathological studies have shown diffuse patchy myocardial fibrosis that may favor ventricular arrhythmias by a re-entry mechanism or by activity of trigger areas. Another arrythmogenic substrate results from small (millimeter) ischemic areas (contraction band necrosis) created by repeated abnormal vasospasm of small coronary arteries and arterioles mimicking Raynaud’s phenomenon of the myocardium.3
The most frequent conduction abnormalities in SSc are P-R interval prolongation (first-degree heart block), left anterior fascicular block, right and left bundle branch block, and nonspecific intraventricular conduction defects. With the same pathophysiological substrate, the most common arrhythmias occurring in SSc are premature ventricular contractions in the form of couplets multifocal, premature atrial contractions, and supraventricular tachycardia. Particular attention must be directed to sustained ventricular tachycardia that represents a poor prognostic factor for sudden death.
In SSc, the incidence of sudden cardiac death is higher than that in the general population. Postmortem studies of SSc patients who die suddenly show a history of ventricular arrhythmias, documented ventricular tachycardias, and frequent multifocal premature ventricular contractions.4 Currently, there is no evidence that drug therapy lowers mortality in SSc patients. In fact, the outcome may be even worse with treatment because of a proarrythmic effect of drugs; the use of drugs, however, may represent a marker of severity of arrhythmia rather than a cause of increased mortality. Whatever the basis for this association, these data suggest that use of anti-arrhythmics in SSc patients should be closely monitored with repeated ambulatory electrocardiography.
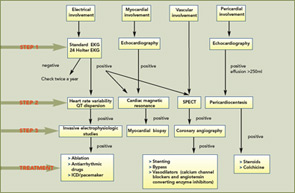
Diagnosis
1) Standard 12-lead electrocardiogram (EKG): At baseline, a standard 12-lead EKG with serial studies at intervals is the most simple, inexpensive, and rapid method for initial cardiac evaluation (see Figure 1, p.17). In SSc, an EKG can detect hidden arrhythmias, asymptomatic conduction defects, and previous ischemic defects. In a large EKG study, ventricular ectopy occurred in 67% of patients and was strongly correlated with total mortality and sudden death, independently from other indexes of severity of disease and organ involvement.
2) Holter monitoring: Holter monitoring is a non-invasive method with high predictive value that permits the early detection of cardiac involvement. Analysis of Holter monitoring data can detect arrhythmias and conduction defects not seen on a standard EKG. Patients with significant abnormalities on Holter monitoring should have further investigations (see Figure 1, p.17). In SSc, Holter monitoring may add valuable information about the autonomic nervous system, assessing the heart rate variability and the QT interval. Recently, abnormalities of heart rate variability and increased QT dispersion have been reported in SSc patients. Autonomic dysfunction represents the cardiac vulnerability resulting from an autonomic imbalance and is a marker for arrhythmic death or an indicator of disease severity. We strongly recommend performance of a Holter at least twice a year in patients with SSc.
3) Invasive electrophysiologic studies: Invasive electrophysiologic studies are indicated when spontaneous arrhythmias are frequent and when a serious sustained arrhythmia is suspected. Electrophysiologic studies can detect the mechanism generating the arrhythmias and allow the selection of patients who can be treated by transcatheter ablation; in this selection, the guidelines are similar to those employed in the management for ventricular tachycardia due to coronary disease.5
Treatment
At present, the management of cardiac involvement in SSc remains largely empirical and derives mainly from evidence obtained from other diseases. There is no evidence that anti-arrhytmic drug therapy lowers mortality in SSc patients. Successful treatment with ablation, both surgical cryoablation and catheter ablation, has been reported. Implantation of a cardioverter defibrillator and/or a pacemaker may be a treatment of choice for patients with life-threatening ventricular arrhythmias and conduction defects.
Myocardial Piece of the Puzzle
Primary myocardial involvement in SSc is characterized by patchy fibrotic lesions distributed in both ventricles. Although advanced myocardial fibrosis may lead to congestive heart failure, systolic or diastolic dysfunction can occur early in the disease, before becoming symptomatic years later. When clinically evident, myocardial involvement is recognized as a poor prognostic factor since the overall mortality rate at five years of SSc patients with proven cardiovascular impairment is over 70%. Usually, physical findings are nonspecific and include signs of congestive heart failure. For this reason, a timely diagnosis is highly recommended.
Recently, increased interest has been devoted to left ventricular diastolic dysfunction. Some authors suggest that this type of dysfunction occurs only in SSc patients with systemic and/or pulmonary hypertension, ventricular hypertrophy and pericardial disease. Other authors believe that diastolic abnormalities in SSc reflect primary involvement of the myocardium that may precede systolic dysfunction or may relate to subclinical myocardial involvement occurring even as early as the onset of Raynaud’s phenomenon.6 This frequent symptomless coronary vasospasm may contribute to myocardial dysfunction.
Diagnosis
1) Echocardiography: In SSc, baseline and serial echocardiography with Doppler should be performed to achieve important information about left and right ventricular morphology and function, valvular function, and/or pericardial effusion. Using improved echochardiographic techniques, like tissue Doppler imaging, regional systolic diastolic abnormalities and direct measures of myocardial velocities can be detected, offering the opportunity of more accurate follow up and earlier treatment. One recent study suggests that the presence of diastolic dysfunction in SSc patients reflects an early asymptomatic myocardial fibrosis, identifying those patients with higher risk for cardiac impairment. Left ventricular diastolic dysfunction is expressed by an inverted E/A ratio that represents early and late ventricular filling during atrial contraction.7 Recently, the use of TDI techniques may improve the detection of heart involvement showing the depression of left and right ventricular systolic and left ventricular diastolic function.8
2) Magnetic resonance imaging (MRI): MRI is superior to other techniques for the assessment of ventricular size, function, and mass because it is non-invasive and has high spatial resolution, high reproducibility, and low inter-observer and intra-observer variability. High-resolution perfusion MRI techniques can identify small subendocardial defects and may allow a non-invasive determination of coronary reserve and the evaluation of fibrotic myocardium compared with viable tissue. Two recent studies indicate that MRI may detect subclinical right heart involvement and can identify myocardial fibrosis in a significant percentage of SSc patients.9 MRI is a valuable tool but the high cost still limits its use.10
3) Myocardial biopsy (MB): In clinical practice, the use of MB is limited by invasiveness of the procedure and high rate of sampling errors as shown by its relatively low diagnostic yield compared with that of autopsy. MB is indicated when there is clinical concern for the presence of other cardiac pathologies that might overlap with SSc. An immunohistological study of MB revealed myocarditis and an increased amount of fibroblast, suggesting that more frequent use of MB might lead to more differentiated therapies. In fact, in this study, an increased amount of AS02-positive fibroblast was found in the myocardium of both SSc patients in comparison with the controls, suggesting that fibroblasts are over-represented in the SSc myocardial tissue of patients in comparison with other myocardial diseases. However, the utility of MB is significantly limited by risks and the possibility of sampling error due to patchy heart involvement.11
Treatment
Early treatment with vasodilators such as calcium channel blockers and angiotensin converting enzyme inhibitors has been shown to be beneficial on myocardial perfusion and function and may help to limit the progression of major life-threatening complication of the disease. Up to now, no antifibrotic therapy has been shown to preview or control myocardial fibrosis.
Vascular Piece of the Puzzle
In SSc, despite of the prominence of vascular abnormalities and documented ischemia, the incidence of coronary artery disease does not seem different from that of the general population.12 Characteristic SSc vascular lesions result in major impairment of the microcirculation. Histological examinations reveal concentric intimal hypertrophy associated with fibrinoid necrosis of intramural coronary arteries. In addition, vasospasm of the small coronary arterioles mimicking a myocardial Raynaud’s phenomenon induce contraction band necrosis that are ischemic tiny fibrotic areas of the myocardium. Two recent studies using enhanced transthoracic Doppler before and after adenosine infusion have confirmed the reduction of coronary reserve in SSc patients without clinical evidence of cardiac involvement. The functional and structural abnormalities of the small coronary circulation are now considered the first pathologic step of the disease that leads to late fibrotic patchy ischemic lesions.
Diagnosis
1) Single photon emission computed tomography (SPECT): The acquisition of perfusion scintigraphy using gated SPECT is an important development of nuclear medicine. Gated SPECT allows the automated calculation of left ventricular ejection fraction (LVEF) and the assessment of regional function using perfusion images. A good correlation exists between resting gated SPECT and other imaging techniques for the calculation of LVEF and the assessment of regional wall motion and thickening. The simultaneous assessment of perfusion and function is helpful for the diagnostic and prognostic assessment of patients with chronic coronary artery disease like SSc patients.
The addition of functional data to stress and rest perfusion images significantly improves their specificity and reduces the uncertainty in test interpretation, with a better separation of normal from abnormal studies SPECT allows the assessment of myocardial perfusion, providing evidence of associated reversible ischaemia. Using pharmacological stress (dipyridamole) with SPECT, decreased heart perfusion was observed in 82% of SSc patients.13 This technique is important in differentiating myocardial stunning from inducible ischemia or necrosis, using the endothelium-dependent vasodilating effect of dipyridamole. SPECT is considered relatively safe, inexpensive, and easy to perform. SPECT can be applied in scintigraphy with thallium-201 and with perfusion markers like Tc-99m. We recommend performance of SPECT as a baseline exam even in asymptomatic patients.
2) Coronary angiography: Coronary angiography offers the most accurate visualization of the coronary arteries and remains the gold standard when a stenotic coronary lesion is suspected. Angiography is not recommended for screening asymptomatic patients.14
Case report
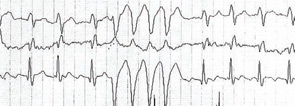
A 53-year-old SSc patient with esophageal and muscle involvement (weakness, elevated CK) developed dyspnea on exertion. Pulmonary function tests and high-resolution computed chest tomography were normal. Cardiac color-Doppler echocardiography PAP was normal, but a left ventricular asynergy with normal ejection fraction (56%) was detected. Cardiac scintigraphy demonstrated an apical–septal area of inducible ischemia. Coronary angiography was performed demonstrating a slow contrast washout representing a microcirculation pathology with no occlusions of the extramural coronary arteries. Cardiac Holter showed the presence of 7.567 monomorphic ventricular extrasystolic beats with 237 pairs and 13 runs of bigeminy despite antiarrhythmic drug (amiodarone) therapy (see Figure 2, p. 17). For this reason, an intracardiac defibrillator (ICD) was implanted.
Six months later, the analysis of the ICD showed that it has been activated with deliverance of four shocks to stop episodes of malignant ventricular tachycardia. This case shows that a prompt diagnosis of arrhythmia and the implantation of an ICD can prevent sudden cardiac death in an SSc patient and this clearly illustrates the value of a comprehensive diagnostic work-up and the benefits of an aggressive patient management.
Pericardial Piece of the Puzzle
Pericardial effusion is a harbinger of a poor prognosis and is more frequent in patients with the diffuse subset of the disease.15,16 Pericardial effusion is also a useful predictor of scleroderma renal crisis.17 In autopsy studies, SSc patients commonly have asymptomatic pericardial abnormalities with a prevalence estimated from 33% to 72%. Recently, it has been reported that pericardial abnormalities are associated with echocardiographically defined pulmonary arterial hypertension (PAH).18
Diagnosis
1) Echocardiography: Echocardiography is the most widely used imaging technique in the evaluation of suspected pericardial disease. Transthoracic echocardiography represents the gold standard for diagnosis of pericardial abnormalities, demonstrating the location and amount of even minimal pericardial effusion. The echocardiogaphic features of pericardial effusion are the pericardial layer separation with an echo-free space and the decrease in the parietal pericardial motion.
2) Pericardiocentesis and biopsy: Pericardiocentesis and biopsy are limited by their invasiveness. Cytology, bacteriology, and virology may be performed on the fluid and can be of important diagnostic value. If tamponade develops, emergency pericardiocentesis is needed.
Treatment
Low-dose steroids may be helpful for the control of pericardial involvement. The addition of colchicine can be an effective treatment for acute and recurrent pericarditis. This combination may be helpful in preventing recurrences in almost 90% of cases.19
Diagnosis and Management
Clinical signs suggesting cardiovascular involvement in SSc are often nonspecific. The first step in practice is to obtain a detailed history, a thorough physical examination and a standard EKG. Thereafter, the baseline evaluation consists of a 24 Holter monitoring, Doppler echocardiography, and SPECT. Assessment of Pro-BNP may be useful for the diagnosis of cardiomyopathy because its concentration is altered in patients with myocardial structural impairment even if asymptomatic: usually, pro-BNP levels are used to monitor patients with PAH.
If baseline studies are normal and the patient has no complaints, we recommend a biannual assessment with Holter and Echo-Doppler (See Figure 1, above). If Holter monitoring provides evidence of arrhythmias or conduction defects, further investigations are necessary and should be guided by the arrhythmic pattern. If the echocardiogram reveals cardiac hypertrophy, a reduced ejection fraction, or a diastolic dysfunction (inverted E/A ratio), more invasive investigations like left- and right-heart catheterization or angiography are needed. If SPECT reveals areas of inducible ischemia, coronary angiography should be performed.
Conclusion
Cardiac findings—ranging from fatal arrhythmias to congestive heart failure—remain serious manifestations of SSc and important sources of morbidity and mortality. These manifestations, which may reflect electrical, vascular, and myocardial pathology, are frequently asymptomatic and can occur alone or together. The rheumatologist must therefore confront the puzzle of cardiac involvement in SSc with a broad array of diagnostic approaches and, by determining which piece of the puzzle is jeopardizing the patient’s life, apply the right treatment. Hopefully, with a more aggressive approach and development of new modalities of treatment, improvements in patient outcome will occur and thereby change the course of this dangerous and puzzling complication of the disease.
Dr. Kaloudi is in the Division of Rheumatology at the University of Florence. Dr. Matucci Cerinic is professor of rheumatology and medicine and director of Division of Medicine and Rheumatology at the University of Florence.
References
- Kahan A, Allanore Y. Primary myocardial involvement in systemic sclerosis. Rheumatology. 2006;45 (Suppl. 4):14-17.
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med. 1988;84:1007-1015.
- Ranking AC. Arrhythmias in systemic sclerosis and related disorders. Card Elecrtophysiol Rev. 2002;6:152-154.
- James TN. De Subitaneis Mortibus VIII: Coronary arteries and conduction system in scleroderma heart disease. Circulation. 1974; 50:844-856.
- Rankin AC, Osswald S, McGovern BA, Ruskin JN, Garan H. Mechanism of sustained monomorphic ventricular tachycardia in systemic sclerosis. Am J Cardiol. 1999;83:633-636.
- Valentini G, Vitale DF, Giunta A, et al. Diastolic abnormalities in systemic sclerosis: Evidence for associated defective cardiac functional reserve. Ann Rheum Dis. 1996;55:455-460.
- Armstrong GP, Whalley GA, Doughty RN. Left ventricular function in scleroderma. Br J Rheumatol. 1996;35:983-988.
- Bezante GP, Rollando D, Sessarego M, et al. Cardiac magnetic resonance imaging detects subclinical right ventricular impairment in systemic sclerosis. J Rheumatol. 2007;34:2431-2437.
- Vignaux O, Allanore Y, Meune C, et al. Evaluation of the effect of nifedipine upon myocardial perfusion and contractility using cardiac magnetic resonance imaging and tissue Doppler echocardiography in systemic sclerosis. Ann Rheum Dis. 2005;64:1268-1273.
- Liangos O, Neure L, Kühl U, et al. The possible role of myocardial biopsy in systemic sclerosis. Rheumatology. 2000;39:674-679.
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography during routine care: A controlled study of 100 consecutive patients. Arthritis Rheum. 2008;58:1803-1809.
- Matucci-Cerinic M, Fiori G, Grenbaum E, Shoenfeld Y. Macrovascular disease in systemic sclerosis. In: Clements PJ, Furst DE. Systemic Sclerosis, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2004; 241-248.
- Ishida R, Murata Y, Sawada Y. Thallium-201 myocardial SPECT in patients with collagen disease. Nucl Med Commun. 2000;21:729-734.
- Akram MR, Handler CE, Williams M, et al. Angiographically proven coronary artery disease in scleroderma. Rheumatology. 2006;45:1395-1398.
- Langley RL, Treadwell EL. Cardiac tamponade and pericardial disorders in connective tissue diseases: Case report and literature review. J Natl Med Assoc. 1994;86:149-153.
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion a case report. Angiology. 2001; 52:59-62.
- Steen VD, Medsger TA Jr, Osial TA Jr, Ziegler GL, Shapiro AP. Factors predicting development of renal involvement in progressive systemic sclerosis. J Med. 1984;76:779-786.
- Fischer A, Misumi S, Curran-Everett D, et al. Pericardial abnormalities predict the presence of echocardiographically defined pulmonary arterial hypertension in systemic sclerosis-related interstitial lung disease. Chest. 2007;131:988-992.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: Results of the Colchicine for acute pericarditis trial. Circulation. 2005;112:2012-2016.
