The field of rheumatology is witnessing a remarkable revolution as the prospects for patients with rheumatoid arthritis (RA) continue to improve dramatically. This change results from two main factors: 1) the more effective utilization of older disease modifying antirheumatic drugs (DMARDs) such as methotrexate alone or in combination with recently available targeted therapies; and 2) a landmark shift in therapeutic strategy. Several trials have clearly shown that early achievement of low disease activity through DMARD use has a strong positive outcome on the disease course. By its nature, this approach necessitates the assessment of disease activity by objective, well-validated measures as well as frequent adjustment of therapy to push toward remission.
Rheumatologists now know the importance of treating early disease; however, the criteria for RA are undergoing changes. The purpose of these revised criteria is increased sensitivity and specificity to diagnose RA in an early phase of disease.
Why Defining RA Is Difficult
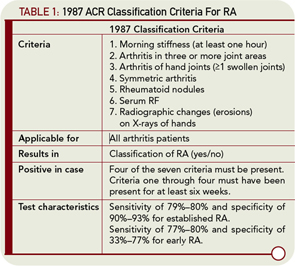
Why is it difficult to define early RA? Ask the average rheumatologist to define RA, and he or she will provide answers like “symmetric polyarthritis of small joints combined with morning stiffness, presence of rheumatoid factor (RF), or anticitrullinated peptide antibodies (ACPA), or combined with bone erosions on X-rays.” It is clear that these views mirror the 1987 ACR criteria for RA and demonstrate the extent to which our thinking on RA is inextricably connected with these criteria. It is obvious that these criteria do not perform well when diagnosing early RA. This is especially true for patients who have limited joint involvement, negative serologies, or negative radiographs.
The 1987 criteria, developed based on patients with an average disease duration of eight years, contain elements that are associated with disease severity (e.g., erosions and nodules) rather than disease development (see Table 1, p. 23). A moderate diagnostic performance of these criteria in early RA is frequently reported. In these studies, the doctor’s opinion is often used as the gold standard. Summarizing the literature, the sensitivity and specificity of the 1987 criteria for early RA are 77%–80% and 33%–77%, respectively, compared with 79%–80% and 90%–93%, respectively, for established RA.1 These differences in performance characteristics are inherent in the objective for which the 1987 ACR criteria were designed. The aim at the time of their creation was to provide correct classification, distinguishing the syndrome of established RA from other established joint diseases, to ensure that clinical researchers studied homogeneous patient groups. Considering the test characteristics that have been reported in many publications, this aim was achieved.
Emerging evidence on the response of RA to early treatment suggests the existence of a window of opportunity in RA, a time during which effective therapy can lead to long-term benefits in outcomes, including a sufficient reduction in disease activity so that existing criteria for RA may not be met. Because these findings point to the need for early treatment, the goals of the criteria appear to have changed from classification criteria to diagnostic criteria. Classification criteria need to identify patients with RA correctly to minimize misclassification in clinical or epidemiological studies. In contrast, diagnostic criteria should allow the clinician to reach a diagnosis when confronted with a given patient in the clinic. Thus, at the present time, to meet the clinical needs of improving outcomes by prompt DMARD therapy, diagnostic tools are required to identify patients with RA at the earliest stages of disease.
The difficulty in developing criteria for RA can be handled in two ways. First, new criteria can be derived, and these criteria can function as both classification criteria and diagnostic criteria for early RA. Alternatively, the definition of RA ascertained in the 1987 ACR classification criteria can remain unchanged, although methodology can be developed to identify those patients with early arthritis who will develop RA (see Table 2, p. 24). Both options have advantages and disadvantages that will be considered here.
Rheumatologists now know the importance of treating early disease … . The purpose of these revised criteria is increased sensitivity and specificity to diagnose RA in an early phase of disease.
Developing New Criteria
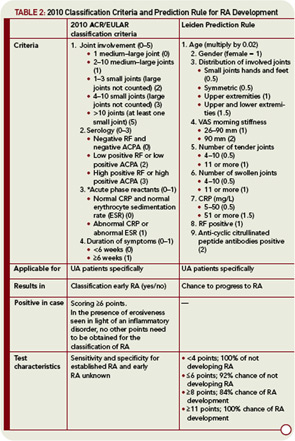
New criteria for RA have been developed by a task force composed of rheumatologists from the United States and Europe, resulting in the ACR/European League Against Rheumatism (EULAR) Classification Criteria.2–4 These new criteria have been developed in two phases. In the first phase, patient characteristics associated with methotrexate treatment were identified using several European and Canadian early arthritis cohorts. Methotrexate prescription during the first year after diagnosis was chosen as an indication of RA. In the subsequent phase of criteria development, the knowledge and experience of expert clinicians were used to reach a consensus for the final criteria set. The combination of data-driven and expert-driven approaches created a set of criteria that is useful and informative—and is supported by a process that incorporates the “gut feelings” from rheumatologists.
The ACR/EULAR criteria for RA are meant to be applied to persons with undifferentiated inflammatory arthritis (UA).2,4 They can be fulfilled in two ways—either by erosiveness (e.g., joint erosions) on X-rays, or by having a combination of six or more points from the following variables: number of involved joints, localization of involved joints (large or small joints), symmetry, duration of synovitis, acute phase reactants, and RF and ACPA that are both weighed for their level (see Table 2, p. 24). Importantly, this construct indicates that if another definable disease better explains the presence of synovitis, the new criteria for RA should not be applied. This statement implies that, in its new definition, RA is a disease of exclusion that should not be diagnosed if other criteria can be fulfilled as well. As such, this definition is different from ACR criteria for other rheumatologic conditions, in which the absence of fulfilling criteria for other diagnoses are not part of the requirements.
The new criteria have not given exact definitions for some of the variables, a situation that may lead to confusion or contention. First, according to the new criteria, if a patient has joint erosions in the context of an inflammatory condition, he or she can be classified as having RA without fulfilling any of the other criteria. Erosive disease is not defined. Is an erosion defined as a broken cortex? Or is a subchondral cyst with an intact cortex, which may precede the development of a lesion with broken cortex, identified as an erosive state as well? Is the localization of erosiveness relevant? In addition, is one erosion enough for the definition of RA, or should a number of erosions be present to be sure that true erosive disease is present?
There are recent data on the prognostic value of baseline erosions in patients presenting with UA. Thabet et al show that the presence of one erosive joint has a limited predictive value for development of RA, according to the ACR criteria and persistent disease.5 The presence of two, three, or four erosive joints had an equivalent positive predictive value; if five or more erosions were present, the predictive ability was further increased. From these data, one can conclude that the term erosive disease would entail the presence of at least two erosive joints. Surprisingly, the presence of two erosive joints at first presentation to the early arthritis clinic is no guarantee of a persistent disease course. Of patients with two erosions, 32% achieved a spontaneous remission.4
These data are in contrast to the significance of baseline erosions in patients with RA (by the 1987 ACR criteria); in these patients, the presence of erosions at baseline is the most powerful prognostic factor for a destructive disease course. This finding underlines the notion that UA contains a much more heterogeneous set of patients than established RA. If the findings of Thabet et al can be replicated in another set of UA patients, this information might be considered in the definition of erosiveness. Indeed, it may raise the question as to whether the presence of erosiveness in patients with early UA is so specific and predictive that no other criteria need to be met to diagnose RA.
The second variable that needs to be clarified in the new criteria is the definition of a high level of RF and a high level of ACPA. There are some data that support the notion that high levels of these autoantibodies have an increased specificity and predictive value compared with low levels. An important issue relates to the appropriate cut off for a high level of these autoantibodies. Establishing a working definition could be problematic because of the variety of available methods for autoantibody measurement. The most common methods are ELISAs. These assays are characterized by differences in performance features such as the standard curves, cut-off values for positivity, and maximal levels. For instance, some RF kits have a reference for positivity of >5 units, whereas in other kits this is 25 units. This means that a level of ≥50 is a moderately elevated level using the latter kit but is a markedly increased level in the former kit. Moreover, the day-to-day variation in assay performance is often considerable. A modest correlation between RF levels determined with different ELISAs and nephelometers in the same sera has been described.6 Differences in identifying a high RF or ACPA level could therefore complicate the application of the new ACR/EULAR criteria and lead to differences in the readiness to diagnose RA between sites.
Another question about the new ACR/EULAR criteria is whether they are entirely novel or influenced by the definition of RA embodied in the 1987 ACR criteria. To prevent circular reasoning, the outcome of the data-driven phase of the criteria development was not the expert’s opinion on the diagnosis of RA, but treatment of a UA patient with methotrexate within the first year. It is not clear whether this outcome really reduces the influence of circular reasoning. Generally, rheumatologists will prescribe methotrexate on the basis of their expert opinion that a patient has RA; however, having worked with the 1987 ACR criteria for about 20 years, clinicians may, consciously or unconsciously, refer to these criteria in their decision making.
Treatment with methotrexate as an outcome for assessing diagnosis also harbors the risk of misclassification on both sides. Patients with early UA who have another form of persistent arthritis (e.g., psoriatric arthritis) may be treated with methotrexate and would be classified in the RA group. Conversely, patients with characteristics of RA may have contraindications to, or side effects from, methotrexate and would be treated with another DMARD; these patients could be incorrectly classified in the non-RA group. It is hoped that, by the analysis of large numbers of patients, these effects can be leveled out. Nevertheless, these examples point to the difficulty of finding an appropriate gold standard for the diagnosis of early RA.
Another example of the difficulty in eliminating circular reasoning relates to the discussion on the inclusion of morning stiffness during the experts’ opinion phase in the development of the new criteria. Although duration of morning stiffness did not discriminate between the patients who did and did not receive methotrexate within one year of symptom onset, many experts felt that morning stiffness duration is a characteristic specific to RA. One may wonder to what extent this clinical impression is supported by the presence of morning stiffness in the 1987 ACR criteria. Eventually, it was decided not to include morning stiffness in the new set of criteria.
Although it is challenging to choose the best outcome measure for early RA, the 2010 set of ACR/EULAR criteria—which, according to the name, are still classification criteria—has a currently unknown sensitivity and specificity for the diagnosis of early RA. It will be interesting to wait for the 2011 ACR and EULAR meetings when, hopefully, many groups who have used these criteria in their own cohorts or clinical trials will report on their experience, and we can see how the criteria perform in various clinical and research settings.
The Prediction Rule
Given the intricacy and difficulty of deriving totally independent criteria for early RA, another option to identify early RA patients is to develop a predictive tool. For this purpose, the current classification criteria do not need to be changed. A prediction tool of this kind has recently been developed.7 This tool consists of nine variables: age, gender, distribution of involved joints, severity of morning stiffness, number of tender joints, number of swollen joints, C-reactive protein (CRP), RF, and anti-cyclic citrullinated peptide antibodies (see Table 2, p. 24). Intriguingly, these variables are quite similar to those in the 2010 ACR/EULAR criteria and contain elements of arthritis indicating joint pathology, inflammation, and autoantibodies. This prediction rule has been derived from prospective data and did not rely on expert opinion for its development. Although this rule was developed based on the experience of a single early arthritis cohort, it has an adequate discriminative ability and the predictive ability of this model has been validated in multiple worldwide studies.8–13
The prediction rule was derived with the ultimate goal of implementing the approach of personalized medicine. Therefore, the prediction rule results in an absolute chance for an individual patient to develop RA within the first year. In making treatment decisions, this chance can be weighed against individual risk, on the basis of side effects or the patient’s wishes, to aid in optimal decision making.
In contrast to the new ACR/EULAR classification criteria, the prediction rule does not result in a yes/no classification for having the disease or not. Nevertheless, patients can be grouped according to the results of this prediction rule. In the derivation cohort, UA patients with ≤6 points had an absolute chance of not getting RA of 91%; UA patients with ≥8 points had an 84% chance of progressing to RA. In the various replication cohorts, these chances ranged between 70%–92% and 75%–95%, respectively. As such, the cut-off value of ≤6 may point to not getting RA or no indication for DMARD therapy and ≥8 for getting RA or an indication to start DMARD therapy. The main disadvantage of using these cut-off values is that about 20% of the patients cannot be classified if they have a score between 6 and 8.
Efforts to improve this prediction rule using radiological, serological, or genetic information have been unsuccessful thus far.2,14 This result likely relates to the fact that most of these factors are associated with clinical variables that are already in the prediction model. Furthermore, the diagnostic accuracy of the prediction rule is already high, making it hard to get significant improvement.
The outcome of the prediction (i.e., the risk on RA development) is clearly understandable, representing an absolute chance for an individual patient. The disadvantage of using positive and negative predictive values (PPV and NPV) is that these absolute chances depend on the disease prevalence. For the predictive rule, the validation studies were performed in different cohorts with different inclusion and exclusion criteria and with different incidences of RA.8–13 In general, the higher the incidence of RA, the higher the PPV and the lower the NPV. Indeed, in the validation cohorts, some increase in PPV was accompanied with some decrease in NPV and vice versa.6–11 Nonetheless, the overall diagnostic accuracy of the prediction rule was preserved.
Two New Tools for Rheumatologists
In conclusion, two efforts have been undertaken to provide criteria and approaches to identify, early in the course of disease, patients who will have a persistent disease course that, according to the 1987 ACR criteria, is characterized as RA. Experts from the ACR and EULAR have derived new classification criteria applicable to patients with early UA. In advancing the application of these criteria, future work will include a focus on the refinement of the definition of some of the criteria, the assessment of the sensitivity and specificity of these criteria in early RA, and determination of the prognostic performance of these criteria.
In addition to the new criteria, a prognostic tool for predicting RA development in patients with recent onset UA has been derived. This prediction rule does not alter the current definition of RA and does not primarily classify patients; rather, this rule provides the likelihood of an individual patient’s chance to progress towards RA. The predictive accuracy of this model is widely validated. Although the approaches were different (deriving new classification criteria or a prognostic tool), both efforts were driven by the concept that UA patients with an unfortunate prognosis should be identified early. Hopefully these efforts will lead to improved outcomes for patients with early undifferentiated arthritis and provide the foundation for new treatment strategies directed at achieving remission and preventing disease progress, permanent damage, and disability.
Dr. van der Helm-van Mil is head of the outpatient clinic in the department of rheumatology and Dr. Huizinga is chair of rheumatology, both at Leiden University Medical Center in Leiden, The Netherlands.
ACR/EULAR RA Criteria Now Available
The 2010 ACR/EULAR classification criteria for rheumatoid arthritis were published in the September issue of Arthritis & Rheumatism (2010;62:2569-2581) and are available for download at www.rheumatology.org in the Practice Management tab.
References
- Banal F, Dougados M, Combescure C, Gossec L. Sensitivity and specificity of the American College of Rheumatology 1987 criteria for the diagnosis of rheumatoid arthritis according to disease duration: A systematic literature review and meta-analysis. Ann Rheum Dis. 2009; 68:1184-1191.
- Funovits J, Aletaha D, Bykerk V, et al. The 2010 ACR/EULAR classification criteria for rheumatoid arthritis: Methodological report Phase 1. Ann Rheum Dis. 2010; 69:1589-1595.
- Neogi T, Aletaha D, Silman A, et al. The 2010 ACR/ EULAR classification criteria for rheumatoid arthritis: Phase 2 methodological report. Arthritis Rheum. 2010; 62:2582-2591
- Aletaha D, Neogi T, Silman A, et al. 2010 Rheumatoid arthritis classification criteria: An ACR/EULAR collaborative initiative. Arthritis Rheum. 2010;62:2569-2581.
- Thabet MM, Huizinga TW, van der Heijde DM, van der Helm-van Mil AH. The prognostic value of baseline erosions in undifferentiated arthritis. Arthritis Res Ther. 2009; 11:R155.
- Stone R, Coppock JS, Dawes PT, Bacon PA, Scott D. Clinical value of ELISA assays for IgM and IgG rheumatoid factors. J Clin Pathol. 1987;40:107-111.
- van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: How to guide individual treatment decisions. Arthritis Rheum. 2007;56:433-440
- van der Helm-van Mil AH, Detert J, le Cessie S, et al. Validation of a prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: Moving toward individualized treatment decision-making. Arthritis Rheum. 2008;58(8):2241-2247
- Kuriya B, Cheng CK, Chen HM, Bykerk VP. Validation of a prediction rule for development of rheumatoid arthritis in patients with early undifferentiated arthritis. Ann Rheum Dis. 2009 Sep;68(9):1482-1485.
- Tamai M, Kawakami A, Uetani M, et al. A prediction rule for disease outcome in patients with undifferentiated arthritis using magnetic resonance imaging of the wrists and finger joints and serologic autoantibodies. Arthritis Rheum. 2009;61:772-778.
- Bradna P, Soukop T, Bastecj D, Hrncir J. Prediction rule in early and very early arthritis patients. Ann Rheum Dis. 2009;68(Suppl 3): 547.
- Luchikhina El, Karateev DE, Nasonov EL. The use of prediction rule to predict the development of rheumatoid arthritis in russian patients with early undifferentiated arthritis. Ann Rheum Dis. 2008;58:2241-2247.
- Mjaavatten MD, Van der Helm-van Mil AH, Huizinga TW, et al. Validation of a proposed prediction rule for rheumatoid arthritis in a cohort of 188 patients with undifferentiated arthritis. Ann Rheum Dis. 2008;67(Suppl II):477.
- van der Linden MP, van der Woude D, Ioan-Facsinay A, et al. Value of anti-modified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis. Arthritis Rheum. 2009;60:2232-2241.