Historical Aspects
The first application of immunoglobulin therapy dates back to the 19th century when immune sera from vaccinated animals were used for prevention and treatment of tetanus, diphtheria, and other infectious diseases. Not until the 1940s were immunoglobulins isolated from human plasma and purified into the first preparations available for use. With anticipation of the United States’ entry into World War II, the National Research Council approached Dr. Edwin Cohn at Harvard University to produce a readily available blood product for the treatment of shock. Albumin was considered an ideal agent for this purpose. Dr. Cohn therefore developed a fractionation process that used cold alcohol to divide plasma proteins into subfractions, producing large amounts of albumin as well as fractions rich in immunoglobulins. Additional modifications resulted in the creation of a successful large-scale fractionation technique and the first immunoglobulin formulations. This Cohn-Oncley cold alcohol fractionation process is still used today.2
Administration of these early formulations of immunoglobulin was often complicated by systemic reactions likely due to contamination with impurities and complement activation by protein aggregates. In 1982, the World Health Organization provided standards for the manufacturing of IVIg products. According to these standards, IVIg should be derived from a pool of at least 1,000 donors. It should have minimal quantities of aggregates, but should also be at least 90% intact with maintenance of full biological functions. IVIg should contain as little IgA as possible and also be free of infectious agents.3 In 1984, the first commercial product from the Swiss Red Cross, Sandoglobulin (Sandoz/Novartis), was licensed for use in the United States. Subsequent refinements to the original Cohn cold alcohol fractionation technique, including additives to prevent IgG re-aggregation, have made newer intravenous preparations safe and well tolerated.
Not All Preparations Are Equivalent
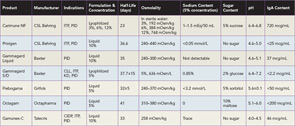
Several preparations of IVIg are now licensed and available in the United States, and selecting the best product for a patient can be challenging (See Table 1). Product features that are important to consider include sugar content, sodium concentration, volume load, osmolality, and amount of IgA. The approaches used to minimize formation of IgG aggregates and stabilize preparations account for most of the variations in composition. Sugars, such as sorbitol, maltose, and sucrose, or amino acids are added to some formulations to prevent aggregate formation. The pH also affects stability, and at an optimum but acidic pH of 4.0–4.5, IgG molecules are present mostly as monomers and additional stabilizers are not required.4