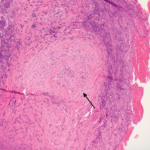
None of this is to say that genetics does not play an important role in the pathogenesis of the anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitides. My heritage is Korean, for example, and by virtue of that fact, I am unlikely to ever be diagnosed with GPA (although I am subject to a host of other, equally inconvenient, diseases).
In 2012, a seminal study by Lyons et al. proved that point. By conducting a genome-wide association study of
1,233 patients in the United Kingdom, they were able to find both major-histocompatibility-complex (MHC) and non-MHC associations for both GPA and microscopic polyangiiits.2 The fact that these associations were disease specific seems to answer, once and for all, whether microscopic polyangiitis is just a forme fruste of GPA: It’s not. But it failed to answer why children won’t inherit a parent’s diagnosis of GPA, but they can inherit their ability to sing.
MHC associations have been identified in a wide range of systemic vasculitides, including giant cell arteritis, Takayasu’s arteritis, IgA vasculitis and Behçet’s disease.3-5,10 None of this information, however, informs my daily practice. In general, I am ignorant to the genetic background of my patients, and even if I had that information in hand, I would not know what to do with it. So why keep looking?
Refractory Rheumatic Disease
Nothing in clinical trials of ANCA-associated vasculitis is more predictable than failure. No matter what the intervention, approximately 15–20% of patients will relapse after two years. The same is basically true for rheumatoid arthritis. No matter how low the bar, at least 20% of patients will fail to respond to any given intervention.
Admittedly, in the post-tumor necrosis factor (TNF) era, even the low bar is set relatively high—most clinical trials enroll patients who have already failed to respond to an anti-TNF; any therapeutic low-hanging fruit has already been plucked. Even given that, there seem to be some patients who don’t respond to drugs as they should. We have all been there—when you find yourself prescribing the next biologic alphabetically rather than based on mechanism. Statistically, 20% of patients will be treated with at least three biologic agents before their doctor finds a drug that works for their disease. In clinical trials, over 70% of patients will fail to have a meaningful response to a second biologic, after having failed to respond to an anti-TNF. What makes these patients different from the rest?