The advent of quality-based healthcare, such as the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), requires rheumatology professionals to demonstrate their practice is based on interventions supported by the best available evidence and that their practice, in turn, provides quality care. These requirements have increased the need for methods to measure and quantify the provision of clinical care and the outcomes of that care.
Evidence developed through typical research methodologies, such as randomized controlled clinical trials, provides excellent support for treatment, but may be difficult to apply to typical clinical care. Rheumatology professionals benefit from research firmly embedded in the realities of clinical care: practice-based evidence.
One form of practice-based evidence is evidence created from the data generated in practice settings. These data are analyzed to describe what is actually occurring in practice and the effect on patients. Practice-based evidence can be used to describe trends in patient care, look at comparative effectiveness of interventions, identify problem areas, help prioritize treatments, address quality improvement and determine where formal research may be needed. This evidence can be used to develop, test and rapidly implement novel measures to define value in rheumatology.1
Gather the Data
Electronic health records (EHRs) have made it much easier to collect data in real time, but significant challenges remain in putting the data to practical use. The data must be aggregated into an interpretable format that can provide real-time continuous information to guide individual patient treatment and clinical quality improvement initiatives. The data collected must be comparable between different clinics so it can be easily collated and compared with established nationwide benchmarks so individual practices can determine their performance for federal quality programs.2
The Rheumatology Informatics System for Effectiveness (RISE) registry is an ideal database to generate practice-based evidence that can be used by rheumatology professionals and researchers alike to improve the care of patients.3
In 2014, the ACR launched RISE as a clinician-friendly method to collect and interpret clinically generated data. The RISE registry collects specific EHR data from member clinics, sends these data to a centralized warehouse where the data are aggregated and analyzed, and then provides the clinic with easily understandable data at the provider and clinic levels in the form of a dashboard. The resulting data can inform patient care, provide evidence of quality care and help management engage in data-driven quality improvement measures.1,2
Clinical Practice Use
How can RISE be used by a busy rheumatology health clinic to improve patient care and monitor and improve quality? Let’s consider the following testimonial from Margarita Fallena, MD, at the Rheumatology Associates, Dallas, who shared some of her experiences with us on using and incorporating the RISE Registry into her clinical practice:
I work in a single-specialty group with a total of 18 rheumatologists. Our practice has been a part of the RISE registry since 2014. The RISE dashboard provides a useful resource to track clinically relevant information related specifically to rheumatic disease outcomes and disease monitoring.
With the information provided in the dashboard, I am able to track my personal performance in specific measures and compare it with previous years, review the data for my practice as a group, and compare our performance to the registry average, which includes data from all other users.
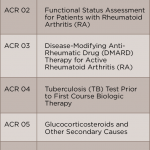
How can this information translate into improved patient care? Reviewing previous performance allows me personally, and as part of a group, to identify areas with opportunities for improvement. Using rheumatoid arthritis as an example, some of the clinically significant measures reported in RISE that are relevant to the care of my patients with rheumatoid arthritis include:
- Documentation of periodic assessments of disease activity measures;
- Tuberculosis screening prior to biologic utilization;
- Vaccination status; and
- Osteoporosis screening.
Comparing my performance individually, and as a group, to other registry users is a valuable tool that can be used in a busy practice to detect deficiencies and implement changes to improve patient outcomes and safety.
RISE Measures & More
RISE offers rheumatology-specific measures for clinicians to gain a deeper understanding of the quality of care provided to their patients. The dashboard distills complex information into easily understandable data points, which show areas of strength and opportunities for improvement. As an added benefit, RISE is Health Insurance Portability and Accountability Act (HIPAA) compliant and a Qualified Clinical Data Registry (QCDR). Rheumatology health providers can submit data for the Quality Payment Program (QPP), directly reporting quality of care data to the Centers for Medicare and Medicaid Services (CMS). It allows rheumatology health providers to participate in QPP via the Merit-Based Incentive Payment System (MIPS) pathway; the program determines each clinician’s CMS reimbursement (positive, neutral or negative) for a calendar year.
RISE allows users to submit for three MIPS categories: Quality, Promoting Interoperability and Improvement Activities. The fourth category, Cost, is calculated by CMS and no data submission is required. Participants may choose to submit to MIPS as a group or individually. RISE users benefit from expert knowledge offered by ACR registry staff and the technical vendor for RISE, FIGmd, regarding MIPS reporting requirements, bonus points for submitting via a QCDR and assistance through the reporting process.
Currently, RISE collects data from more than 1,000 providers from (mostly) community practices of all sizes across the U.S. Participation in RISE is a benefit of ACR and ARP membership; there is no cost to members. Practices typically spend 10–15 hours over the course of six to eight weeks on the implementation process (see Figure 1); time can vary depending on the practice’s EHR. Participants will work with both ACR and FIGmd throughout the process.
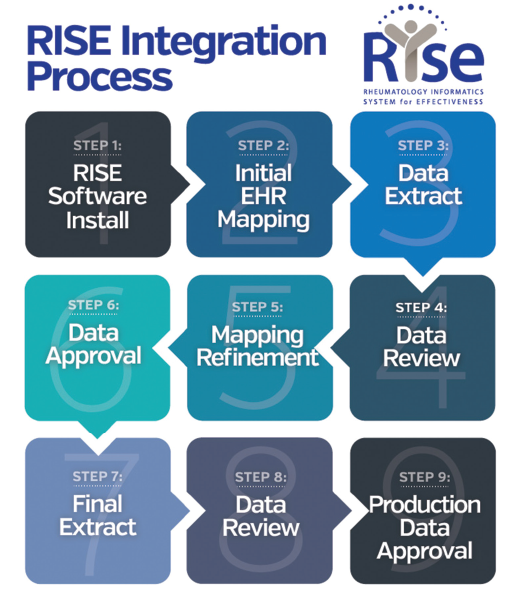
Figure 1. Overview of the process to complete electronic health record connection with the Rheumatology Informatics System for Effectiveness (RISE) registry.
Interested clinicians are encouraged to contact RISE staff to set up a demonstration of the tool and an inside look at the dashboard at the practice’s convenience prior to registering.
Research Use
RISE is also an important tool for researchers interested in practice-based research, because it can be used to look for evolving trends in care, patient outcomes and best practices.2 RISE is a national database that continuously collects data from the EHRs of participating practices. It is a treasure trove of data on clinical practice and has the potential to provide researchers with rich data that can inform evolving research needs.
RISE data go through several levels of cleaning and validation, including the removal of patient identifiers. This allows researchers and clinicians to confidently use RISE data to understand what is actually happening in real-world practices and how patients are affected by the care they are receiving. RISE data available for research currently contains information on approximately 1.5 million patients and 9 million patient encounters.
RISE offers access to data from a diverse patient population: not only the most common diagnoses of rheumatoid arthritis (RA) and osteoarthritis, but also less common disorders, such as Takayasu’s arteritis, dermatomyositis and Behçet’s disease. RISE contains detailed information on patient demographics, such as age, insurance status and geography, and on clinical characteristics, such as diagnoses, medications and treatment outcomes, such as CDAI, RAPID3 and HAQ scores.
Data from RISE are already lending new insights into patient care. Recent ACR/ARHP Annual Meeting abstracts included a discussion on the effect of diabetes on RA-related outcomes, practice variation in prescriptions of non-TNFi biologics and tofacitinib, and whether patients with moderate or high disease activity escalate their RA therapy according to treat-to-target principles.4-6 Overall, researchers using RISE data contributed six abstracts in 2018 and three in 2017.
Get Started
The ACR offers various pathways to accommodate different research and budget needs. Researchers interested in using RISE data are required to submit a formal data use request to the ACR (see Figure 2). All requests are reviewed by ACR staff and a committee of experts to determine feasibility and prevent overlap with other requests. All approved projects using RISE data are completed through a data analytic center with expertise in analyzing EHR data.
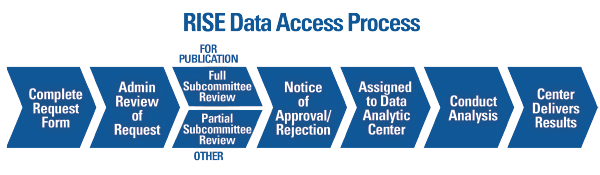
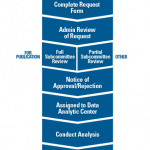
Figure 2. Overview of the process to request and complete a project using data from the Rheumatology Informatics System for Effectiveness (RISE) registry.
The process to review requests for use of RISE data can take two to three months, so if the data will be used for a grant-funded research project, the request should be completed early enough to allow for approval prior to the grant submission. To learn more about the process, visit the RISE for Research section of www.RISEregistry.org. Investigators are also encouraged to contact RISE staff directly at [email protected] to learn more about data that may be available through RISE for their research.
Note: The authors are an inter-professional rheumatology team that consists of four current/past members of the ARP Research Subcommittee, two RISE registry staff members and a member of the ACR Registries and Health Information Technology Committee.
References
- Francisco M, Johansson T, Kazi S. Overview of the American College of Rheumatology’s electronic health record-enabled registry: The Rheumatology Informatics System for Effectiveness. Clin Exp Rheumatol. 2016 Sep–Oct;34(5 Suppl 101):S102–S104.
- Yazdany J, Bansback N, Clowse M, et al. Rheumatology Informatics System for Effectiveness: A national informatics-enabled registry for quality improvement. Arthritis Care Res (Hoboken). 2016 Dec;68(12):1866–1873.
- American College of Rheumatology. RISE for Practices. (n.d.).
- Yun H, Xie F, Chen L, et al. The effect of concomitant diabetes on RA-related outcomes: Results from the ACR’s RISE registry (abstract 223). Arthritis Rheumatol. 2018;70(suppl 10).
- Schmajuk G, Evans M, Kay J, et al. Practice variation in prescriptions of non-TNFi biologics and tofacitinib: Data from the Rheumatology Informatics System for Effectiveness (RISE) registry (abstract 1887). Arthritis Rheumatol. 2018;70(suppl 10).
- Yun H, Chen L, Xie F, et al. Do patients with moderate or high disease activity escalate ra therapy according to treat-to-target principles? Results from the ACR’s RISE registry (abstract 2856). Arthritis Rheumatol. 2018;70(suppl 10).