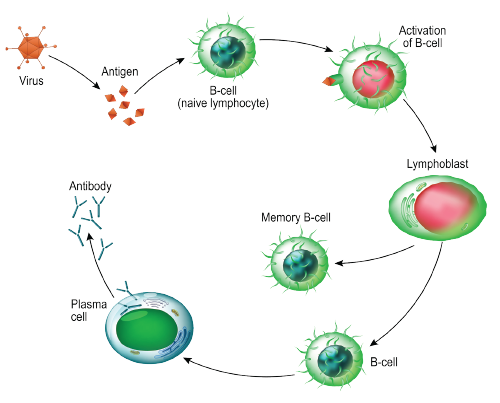
The body uses B cells to produce antibodies that can fight off infection by invading antigens and bacteria. But in rheumatoid arthritis (RA), the immune system produces autoantibodies that work against the body’s proteins to attack joint tissues. Researchers believe this process is helped along by autoreactive B cells that live in bone matter and release proinflammatory cytokines.
As part of a new study, researchers used ribonucleic acid (RNA) sequencing to obtain a comprehensive transcriptome profile of human citrulline-specific B cells in patients with RA.1
The researchers investigated relationships between interleukin (IL) 15α and citrulline-specific B cells. They also studied the capability of B cells from RA patients to produce amphiregulin, a protein that is part of the epidermal growth factor (EGF) family and that stimulates differentiation and growth of cells. The findings, published in the journal Arthritis & Rheumatology, indicate the potential role B cells play in increased differentiation of cells responsible for eroding bone.
“Our results portray B cells as not merely autoantibody producers, but as a source of diverse molecules that can influence proliferation, differentiation and activation of other pathogenic cells types,” wrote Ankit Mahendra, PhD, the study’s lead author and a scientist employed by Sanofi. At the time of the study’s publication, he was a postdoctoral fellow at the University of Houston’s Department of Chemical and Biomolecular Engineering.
The Methods
In this study, the researchers investigated “if there are fundamental differences in the biology of the B cells themselves,” says senior author Navin Varadarajan, PhD, associate professor of chemical and biomolecular engineering at the University of Houston.
The research team compared three groups of cell types: autoreactive anti-cyclic citrullinated peptide (CCP) and rheumatoid factor positive (RA-CCPpos) B cells from RA patients; RA-CCP negative (RA-CCPneg) B cells from RA patients that were not autoreactive; and B cells from healthy donors who produced antibodies against influenza (hemagglutinin-specific/HA) after vaccination.
“We wanted to take an unbiased discovery approach to see what’s different about the biology of these B cells compared [with] B cells from healthy donors,” Dr. Varadarajan says. “We used RNA sequencing to understand differences in basic B cell biology between these different groups.”
Hemagglutinin is a surface protein from influenza the researchers used to identify B cells likely to be reactive to influenza. Similarly, “autoantibodies in rheumatoid arthritis are reactive to citrullinated peptides,” says Dr. Varadarajan. “So you are using the citrullinated peptides to identify B cells that are autoreactive.”
Researchers isolated citrulline- and hemagglutinin-specific B cells using peptide-streptavidin conjugates from peripheral blood of RA patients and healthy controls. With messenger RNA (mRNA) serving as a surrogate for protein expression, the researchers conducted RNA sequencing of the sorted cells to obtain a transcriptome profile.
Key protein molecules were evaluated with aptamer-based proteomic assays and flow cytometry. In vitro functional assays were employed to study how the proteins affect differentiation of osteoclasts and proliferation and migration of synoviocytes, cells inside the synovium that are considered a hallmark of RA pathogenesis.
What They Found
Results showed the RA-CCPpos B cells differentially express IL-15α and genes related to protein citrullination, as well as signaling of cyclic adenosine monophosphate (cAMP), which regulates several immune pathways in RA. This indicates that, unlike RA-CCPneg B cells, RA-CCPpos B cells play a potential role in protein citrullination and inflammation.
Other findings were consistent with previous studies of B cell pathways related to excess production of tumor necrosis factor (TNF), a protein associated with inflammation in RA patients, Dr. Varadarajan says. More surprising was that the receptor for the cytokine IL-15 was present specifically only in the autoreactive B cells from patients with RA.
Designua / shutterstock.com
“They were not on either the healthy B cells or the B cells that were not autoreactive in these RA patients,” Dr. Varadarajan says.
Researchers also discovered new information about amphiregulin, Dr. Varadarajan says. For years EGF ligands have been well chronicled for their role in the development of cancer and RA. “One of the surprising things that came out of our study was that a particular family member, called amphiregulin, was present in the autoreactive B cells” from the donor RA patients but was not present in the proteins from healthy donors, he says.
To further investigate the role of amphiregulin in autoreactive B cells, the team conducted validation studies that indicated the concentration of the protein was higher in blood and the synovial fluid of CCP-positive patients with RA, he says. These elevations spur the proliferation of an aggressive phenotype of fibroblast-like synoviocytes.
“That also confirmed the importance of this particular protein that we found in these autoreactive B cells. Prior to this, we are not aware of any study that shows that B cells are even capable of making [amphiregulin],” Dr. Varadarajan says.
Learning About the Pathways
The amphiregulin discovery is significant because the protein appears to work in concert with anti-citrullinated protein antibodies to cause increased differentiation of multi-nucleated osteoclasts, cells responsible for bone erosion.
“That’s the finding that links everything together,” Dr. Varadarajan says. “The B cells can produce these amphiregulins, and B cells also make these autoantibodies.
“If we take a step back and think globally, we’ve produced a map of what is different between autoreactive B cells and B cells from healthy donors,” he says.
The generated data lay the groundwork for other researchers who want to further investigate B cell pathways in RA and other autoimmune disorders, Dr. Mahendra says.
Catherine Kolonko is a medical writer based in Oregon.
Reference
- Mahendra A, Yang X, Abnouf S, et al. Beyond autoantibodies: Biologic roles of human autoreactive B cells in rheumatoid arthritis revealed by RNA-sequencing. Arthritis Rheumatol. 2019 Apr;71(4):529–541.