The definition of pruritus, more commonly known as itch, was introduced by a German physician named Samuel Hafenreffer in 1660 as an “unpleasant sensation that elicits the desire or reflex to scratch.”1 Chronic pruritus affects approximately 15–20% of the U.S. population and accounts for more than 7 million outpatient visits per year in the U.S.2 In addition to its high prevalence, chronic pruritus affects multiple quality of life (QoL) parameters, including mood, concentration, eating habits, sexual function and sleep.3-5 Indeed, QoL studies have shown equivalence in terms of impact of chronic pain and chronic itch on QoL measures.6
Despite the high burden of itch-associated conditions on both society and the individual, itch as a medical problem remains an overlooked epidemic because the etiology of itch remains poorly understood. Even 354 years after Hafenreffer initially defined pruritus, treatments remain limited, with no FDA-approved medications for use in humans.
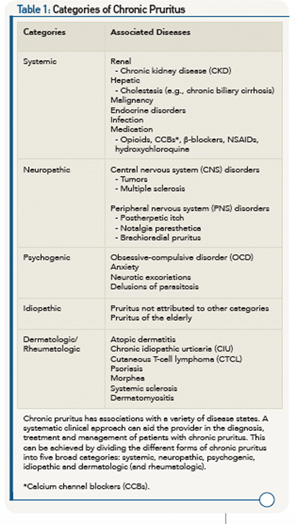
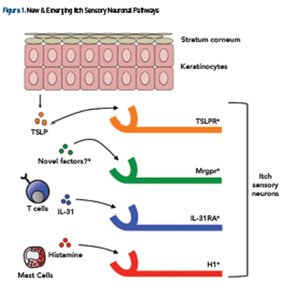
It’s widely appreciated that chronic pruritus underlies multiple dermatologic conditions, such as atopic dermatitis (AD), contact dermatitis and psoriasis. Beyond the skin, pruritus is associated with a variety of systemic medical conditions, including, but not limited to, chronic kidney disease (CKD), liver dysfunction, malignancy, various infections and even psychiatric disease (see Table 1). Recent scientific advances have revealed previously unrecognized itch-specific pathways that are regulated by particular mediators, including neurotransmitters, pharmacologic agents and inflammatory cytokines (see Figure 1).7,8 Although off-label treatments can be employed, more investigation is needed to develop definitive therapeutics to appropriately target chronic pruritus. Recent scientific discoveries have led to a greater understanding of the mechanisms underlying the sensation of itch, which will invariably inform the design of novel treatments in the future.
Pathophysiology of Itch
Classically, itch was viewed as a mild form of pain. As a result, the prevailing wisdom was that the same neurologic pathways mediated both pain and itch. The identification of histamine-responsive neurons in the periphery revealed for the first time that distinct itch-sensitive, pain-independent pathways were present.9,10 However, it was widely appreciated that antihistamines, although effective in certain forms of itch (e.g., urticaria-associated itch), were ineffective in the treatment of many forms of chronic pruritus. These clinical observations provoked the hypothesis that perhaps histamine-independent pathways were present.
Acute itch, as seen in response to arthropod bites & poison ivy, is transient in nature. In contrast, chronic itch is highly debilitating & its etiology is often difficult to define.
Indeed, in 2009 Liu et al identified the Mas-related G protein-coupled receptor (Mrgpr) as a novel family of histamine-independent itch receptors expressed in the dorsal root ganglia (DRG).11 Subtypes of Mrgprs have been identified in both mice and humans.12 Further, MrgprA3 is activated by the drug chloroquine, which has been associated with the development of itch in patients.11 Beyond the peripheral nervous system (PNS), Sun et al identified the first receptor that mediates itch in the central nervous system (CNS), namely gastrin-releasing peptide receptor (GRPR).13 This seminal discovery identified that itch can be regulated both in the periphery, where exogenous pruritogenic stimuli may be encountered, as well as centrally in the spinal cord itself.
Collectively, these emerging studies demonstrate that the factors and pathways regulating itch are much more heterogeneous than previously recognized (see Figure 1). This new scientific paradigm of itch biology may help us to better understand and conceptualize the different forms of itch and their potential treatments.
Emerging studies demonstrate that the factors & pathways regulating itch are much more heterogeneous than previously recognized.
Categories of Itch
Acute itch, as seen in response to arthropod bites and poison ivy, is transient in nature. In contrast, chronic itch is highly debilitating and its etiology is often difficult to define. Chronic itch, defined as lasting greater than six weeks, accompanies a number of inflammatory skin diseases, such as AD and psoriasis, as well as systemic conditions, such as CKD and cholestasis.14 The presentation of chronic itch can be divided into five different clinical categories to help organize the clinical approach, diagnosis and treatment:15
- Systemic itch;
- Neuropathic itch;
- Psychogenic itch;
- Idiopathic itch; and
- Dermatologic itch.
In this review, we briefly outline our current understanding of each category of chronic itch and its potential treatments. Further, we highlight chronic pruritus as a problem in the context of rheumatologic and autoimmune diseases. Although the five categories of chronic itch overlap at times in terms of their pathophysiology and treatments, this structure allows for a systematic and logical clinical approach (see Table 1).
Systemic Itch
The causes of systemic itch are broad and include renal failure, cholestasis and other related hepatic diseases, malignancies, endocrine disorders, systemic infection and exposure to specific drugs. Although a variety of medical conditions underlie chronic itch due to systemic causes, a common theme among these disorders is that a systemic factor is either overproduced or inadequately metabolized and excreted to mediate the itch response. In the following section, we highlight current concepts and treatments regarding a few forms of the most common causes of systemic itch.
CKD is one of the main causes of systemic itch and is often referred to as renal pruritus. Renal pruritus affects up to 58% of patients with renal failure.16 It can be either intermittent or constant and most commonly affects the back, in addition to the head, arms and abdomen.17 Although the pathophysiology is unknown, multiple hypotheses have been suggested. For example, it has been proposed that immune dysregulation underlies renal pruritus, based on the observation that patients respond to immunosuppressive light therapy.18
Beyond the immune system, the accumulation of calcium, magnesium and phosphate due to a lack of renal clearance has also been hypothesized as a contributor. In support of this, symptoms often improve following interventions that normalize electrolytes, such as parathyroidectomy.19
More recently, serotonin and endogenous opioids have also been implicated in renal pruritus.20 Based on these observations, current therapies attempt to target a variety of these underlying processes. In renal pruritus, immunologic treatments include thalidomide, pentoxifylline, doxepin and broadband UVB therapy, which have demonstrated efficacy.21-24 Additionally, selective serotonin reuptake inhibitors (SSRIs), naltrexone and nalfurafine have also been employed to target serotonin and opioid dysregulation.25,26 Gabapentin with dialysis has also been shown to be very effective in multiple studies.27,28 However, universal treatments for renal pruritus remain limited.
Itch is a key determinant of the efficacy of new biologic therapeutics in psoriasis.
Another common systemic cause of chronic pruritus is liver disease. Specifically, diseases resulting in cholestasis are the most common cause of chronic itch in this context.29,30 For example, over 69% of patients with primary biliary cirrhosis report pruritus as a symptom.31 Cholestasis-associated pruritus is widespread and intractable, and can be severe enough to warrant liver transplantation.32 Although the pruritogens are unknown, bile salt and their metabolites are believed to be involved. One hypothesis is that pruritogens secreted into bile are converted in the liver and gut to modulate endogenous serotoninergic and opioidergic systems linked to pruritus.33 Treatment of cholestatic pruritus starts with addressing the etiology of liver disease, including gallstone removal or treatment with cholestyramine, which binds bile salts, or rifampin, which inhibits bile acid production.34,35 Additionally, naltrexone, which targets the opioidergic system, has demonstrated some efficacy.36 More recently, the potent neuronal activator lysophosphatidic acid (LPA) has been found to be elevated in sera of patients with cholestasis and pruritus and represents a potential future drug target.37 Similar to renal pruritus, the mechanisms of cholestatic pruritus and other hepatic etiologies still require further investigation for the development of definitive, targeted treatments.
Importantly, independent of end-organ dysfunction causing systemic chronic pruritus, medications should always be considered potential culprits. It is widely appreciated that opioids, calcium channel blockers (CCBs), β-adrenergic blockers and NSAIDs can induce pruritus.38 Hydroxychloroquine, an antimalarial that is often employed in the treatment of rheumatologic diseases, has also been reported to cause pruritus. As highlighted earlier, recent studies by Liu et al have revealed that this is mediated specifically via activation of Mrgpr on itch sensory neurons.11 Therefore, it’s imperative that patients be evaluated for long-term use of medications that may induce their chronic pruritus. Although we review only a few contributors to systemic pruritus, many etiologies exist. Therefore, when working up chronic pruritus, these should should be considered and have been reviewed extensively elsewhere.15,39,40
Neuropathic Itch
In contrast to systemic chronic itch, which is likely mediated by circulating factors or pruritogens, neuropathic itch derives from pathology along the afferent nervous system.41 The nervous system can be activated either centrally or peripherally. Tumors or degenerative diseases of the CNS (e.g., multiple sclerosis) can lead to centrally mediated mechanisms of pruritus.42
More commonly, neuropathic itch is triggered within the PNS. For example, viral damage to peripheral neurons is thought to mediate postherpetic itch in a manner similar to postherpetic neuralgia.43,44
Another example of peripherally mediated pruritus includes notalgia paresthetica, which presents as chronic, localized itch most often on the central back. Although the exact etiology remains unknown for notalgia paresthetica, it has been theorized that sensory nerve entrapment, involving the posterior rami of the T2 to T6 nerve root, occurs due to degenerative changes in the vertebrae.45 In a similar fashion, brachioradial pruritus manifests as chronic, intermittent pruritus along the arms and is thought to be caused by nerve impingement along the cervical spine.46
For the treatment of neuropathic itch, a variety of neuromodulators are employed empirically. For example, topical capsaicin has been shown to improve symptoms in notalgia parasthetica.47 The mechanism of action of capsaicin is activation of the sensory nerve receptor TRPV1, which leads to depletion of substance P and neuronal desensitization.48 For postherpetic itch and brachioradial pruritus, gabapentin has demonstrated efficacy as well.49 Its mechanism of action is believed to be via binding of the α2δ1 subunit of voltage-dependent calcium channels in dorsal horn postsynaptic cells.50
Other treatments attempted in neuropathic itch include oxcarbazepine, botulinum toxin or, in the case of nerve entrapment, physical therapy and/or surgery.
Psychogenic Itch
Psychogenic pruritus is a diagnosis of exclusion that presents in association with an underlying psychiatric disorder, such as obsessive-compulsive disorder (OCD), anxiety disorder or substance abuse.41 Associated conditions include neurotic excoriations or excoriation disorder, which is now recognized in the DSM-V manual.51 These diseases are strongly influenced by variations in life events and stress. Typical findings include excoriations, erosions, ulcers and scarring in areas of the body that can be easily manipulated by the patient, such as the extensor surfaces and face.52 Delusions of parasitosis, a relatively uncommon psychiatric condition, presents with the patient having a fixed belief that he or she has been infested with arthropods or parasites.53 The patient suffers from these delusions and consciously produces self-inflicted wounds as a result.
The overall approach to the treatment of psychogenic itch is to treat the underlying psychiatric disorder with anxiolytics, antidepressants and/or other neuroleptics. Patients typically need to see their primary physician regularly to develop strong therapeutic rapport. Once the patient is willing, a psychiatric referral is highly helpful, if not essential.
Idiopathic Itch
Idiopathic itch is defined as chronic itch in the absence of specific skin disease and lack of any evidence of underlying disease in the aforementioned categories.54 We include what has been described as pruritus of the elderly within this category because many aging conditions are not necessarily defined as distinct disease entities.55 Although the mechanism is unknown, it has been suggested that chronic generalized pruritus develops in response to gradual loss of skin barrier function, immunosenescence of the skin immune system and sensory neuropathy.56
Initial treatments can include restoring skin barrier function with cream- or ointment-based emollients and gentle atopic skin care. In severe cases, treatment by ‘soak and smear’ with topical steroids as well as steroid-sparing systemic agents such as azathioprine can be helpful.57 Similar to psychogenic itch, this is a diagnosis of exclusion and the astute physician must ensure no underlying systemic or inflammatory conditions are present.
Dermatologic (& Rheumatologic) Itch
Pruritus results most commonly from an inflammatory pathology in the skin. Atopic dermatitis, chronic urticaria, cutaneous T cell lymphoma (CTCL) and autoimmune skin conditions are often associated with chronic pruritus. Understanding the molecular basis of dermatologic itch will likely yield new targeted treatments. Recent studies have demonstrated that inflammatory cytokines, such as thymic stromal lymphopoietin (TSLP) (derived from keratinocytes) and IL-31 (derived from T helper type 2 cells), directly bind itch sensory neurons, suggesting that atopic itch may be targeted therapeutically (see Figure 1).58,59
In chronic idiopathic urticaria (CIU), activation of mast cells has been implicated in the pathogenesis of itch via the binding of IgE onto cell surface FcεRIα receptors.60 Indeed, this has been supported by recent clinical trials demonstrating that omalizumab, an anti-FcεRIα monoclonal antibody, is highly effective in the treatment of CIU-associated itch.61 Collectively, these studies demonstrate that the identification of novel immunologic pathways that mediate itch hold great promise for future treatments (see Figure 1).
In terms of autoimmune and rheumatologic skin disease, psoriasis is the most common and affects 1–3% of the population. It presents with silvery, scaly plaques, generally on extensor surfaces.62 The prevalence of pruritus among patients with psoriasis ranges from 64–97%, and the intensity of itch severity correlates with the development of lesional plaques.63-67 Although T helper type 17 (Th17) cell responses have emerged as dominant mechanisms underlying psoriasis, the definitive pathway by which itch is elicited remains poorly defined.68,69 Notwithstanding this, itch is a key determinant of the efficacy of new biologic therapeutics in psoriasis.
Beyond psoriasis, other autoimmune conditions affecting the skin have been associated with decreased QoL due to pruritus, including morphea, systemic sclerosis and dermatomyositis.70-73 In a recent study, among 959 patients with systemic sclerosis, 42.6% reported pruritus, and its presence was significantly associated with skin and gastrointestinal involvement of disease.74 This suggests that pruritus may be an indicator of systemic disease burden. In the context of cutaneous lupus erythematosus (CLE) and dermatomyositis (DM), a recent study demonstrated that DM has a much stronger association with pruritus than lupus and may help to distinguish between those individuals with CLE and DM.73 Further, improvement of DM disease severity is associated with improvement in pruritus and QoL.75 This is consistent with prior studies demonstrating that pruritus is a significant contributor to QoL in DM patients.76,77
Taken together, these studies demonstrate that the burden of chronic pruritus in rheumatologic diseases is significant and multifaceted. A unifying theme of pruritus associated with autoimmune disease is that it is another measure of disease activity and adequate treatment of the underlying process is essential to improvement of the symptom.
Summary
In summary, we have highlighted five broad categories of itch: 1) systemic, 2) neuropathic, 3) psychogenic, 4) idiopathic and 5) dermatologic. Itch associated with rheumatologic disease is generally manifested in the latter category. The potential causes of chronic pruritus in most patients are multifactorial.
Overall, stratifying the potential causes of pruritus allows for a systematic approach, aiding the provider in developing a better understanding of the underlying diseases, pathophysiology and potential treatments. Emerging studies are revealing previously unrecognized pathways that regulate itch and hold great promise for new, targeted treatments.

Shivani V. Tripathi, MD, is a clinical research fellow in the Division of Dermatology at Washington University School of Medicine in St. Louis, Mo.

Brian S. Kim, MD, MTR, is assistant professor of medicine (dermatology) in the Department of Medicine/Division of Dermatology at Washington University School of Medicine in St. Louis, Mo.
References
- Rost GA. [Samuel Hafenreffer, author of the first textbook on dermatology in German speaking countries]. Z Für Haut- Geschlechtskrankh. 1953 Apr 1;14(7):227–230.
- Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: A population-based cohort study. Acta Derm Venereol. 2013 Sep 4;93(5):532–537.
- Dalgard F, Lien L, Dalen I. Itch in the community: Associations with psychosocial factors among adults. J Eur Acad Dermatol Venereol. 2007 Oct;21(9):1215–1219.
- Yamamoto Y, Yamazaki S, Hayashino Y, et al. Association between frequency of pruritic symptoms and perceived psychological stress: A Japanese population-based study. Arch Dermatol. 2009 Dec;145(12):1384–1388.
- Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol. 2007;87(4):295–298.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: The skin equivalent of pain. Arch Dermatol. 2011 Oct;147(10):1153–1156.
- Ji R-R. Recent progress in understanding the mechanisms of pain and itch. Neurosci Bull. 2012 Apr;28(2):89–90.
- Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: The molecules, cells and circuits of itch. Nat Neurosci. 2014 Feb;17(2):175–182.
- Schmelz M, Schmidt R, Bickel A, et al. Specific C-receptors for itch in human skin. J Neurosci. 1997 Oct 15;17(20):8003–8008.
- Tani E, Ishikawa T. Histamine acts directly on calcitonin gene-related peptide- and substance P-containing trigeminal ganglion neurons as assessed by calcium influx and immunocytochemistry. Auris Nasus Larynx. 1990;17(4):267–274.
- Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009 Dec 24;139(7):1353–1365.
- McNeil B, Dong X. Mrgprs as itch receptors. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press; 2014 [cited 2014 Aug 25]. Available from http://www.ncbi.nlm.nih.gov/books/NBK200929.
- Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007 Aug 9;448(7154):700–703.
- Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: A position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87(4):291–294.
- Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013 Apr 25;368(17):1625–1634.
- Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010 Aug;5(8):1410–1419.
- Berger TG, Steinhoff M. Pruritus and renal failure. Semin Cutan Med Surg. 2011 Jun;30(2):99–100.
- Hsu MM-L, Yang CC. Uraemic pruritus responsive to broadband ultraviolet (UV) B therapy does not readily respond to narrowband UVB therapy. Br J Dermatol. 2003 Oct;149(4):888–889.
- Narita I, Iguchi S, Omori K, et al. Uremic pruritus in chronic hemodialysis patients. J Nephrol. 2008 Apr;21(2):161–165.
- Greaves MW. Pathogenesis and treatment of pruritus. Curr Allergy Asthma Rep. 2010 Jul;10(4):236–242.
- Silva SR, Viana PC, Lugon NV, et al. Thalidomide for the treatment of uremic pruritus: A crossover randomized double-blind trial. Nephron. 1994;67(3):270–273.
- Mettang T, Krumme B, Bohler J, et al. Pentoxifylline as treatment for uraemic pruritus—An addition to the weak armentarium for a common clinical symptom? Nephrol Dial Transplant. 2007 Sep;22(9):2727–2728.
- Pour-Reza-Gholi F, Nasrollahi A, Firouzan A, et al. Low-dose doxepin for treatment of pruritus in patients on hemodialysis. Iran J Kidney Dis. 2007 Jul;1(1):34–37.
- Schultz BC, Roenigk HH. Uremic pruritus treated with ultraviolet light. JAMA 1980 May 9;243(18):1836–1837.
- Peer G, Kivity S, Agami O, et al. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet. 1996 Dec 7;348(9041):1552–1554.
- Kumagai H, Ebata T, Takamori K, Muramatsu T, et al. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010 Apr;25(4):1251–1257.
- Gunal AI, Ozalp G, Yoldas TK, et al. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004 Dec;19(12):3137–3139.
- Razeghi E, Eskandari D, Ganji MR, et al. Gabapentin and uremic pruritus in hemodialysis patients. Ren Fail. 2009;31(2):85–90.
- Bergasa NV. Pruritus in chronic liver disease: Mechanisms and treatment. Curr Gastroenterol Rep. 2004 Feb;6(1):10–16.
- Bunchorntavakul C, Reddy KR. Pruritus in chronic cholestatic liver disease. Clin Liver Dis. 2012 May;16(2):331–346.
- Rishe E, Azarm A, Bergasa NV. Itch in primary biliary cirrhosis: A patient’s perspective. Acta Derm Venereol. 2008;88(1):34–37.
- Bolier AR, Peri S, Oude Elferink RPJ, et al. The challenge of cholestatic pruritus. Acta Gastro-Enterol Belg. 2012 Dec;75(4):399–404.
- Imam MH, Gossard AA, Sinakos E, et al. Pathogenesis and management of pruritus in cholestatic liver disease. J Gastroenterol Hepatol. 2012 Jul;27(7):1150–1158.
- Connolly CS, Kantor GR, Menduke H. Hepatobiliary pruritus: What are effective treatments? J Am Acad Dermatol. 1995 Nov;33(5 Pt 1):801–805.
- Li T, Chiang JYL. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2005 Jan;288(1):G74–G84.
- Bolognia J, Jorizzo JL, Rapini RP, editors. Dermatology. 2nd ed. St. Louis, Mo.: Mosby/Elsevier; 2008. 2 p.
- Kremer AE, Martens JJWW, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010 Sep;139(3):1008–1018, 1018.e1.
- Reich A, Ständer S, Szepietowski JC. Drug-induced pruritus: A review. Acta Derm Venereol. 2009;89(3):236–244.
- Steinhoff M, Cevikbas F, Ikoma A, Berger TG. Pruritus: Management algorithms and experimental therapies. Semin Cutan Med Surg. 2011 Jun;30(2):127–137.
- Cassano N, Tessari G, Vena GA, et al. Chronic pruritus in the absence of specific skin disease: An update on pathophysiology, diagnosis, and therapy. Am J Clin Dermatol. 2010 Dec 1;11(6):399–411.
- Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008 Feb;21(1):32–41.
- Koeppel MC, Bramont C, Ceccaldi M, et al. Paroxysmal pruritus and multiple sclerosis. Br J Dermatol. 1993 Nov;129(5):597–598.
- Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011 Jun;30(2):87–92.
- Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008 Jan;9(1 Suppl 1):S10–S18.
- Shin J, Kim YC. Neuropathic itch of the back: A case of notalgia paresthetica. Ann Dermatol. 2014 Jun;26(3):392–394.
- Veien NK, Laurberg G. Brachioradial pruritus: A follow-up of 76 patients. Acta Derm Venereol. 2011 Mar;91(2):183–185.
- Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: Vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol. 1995 Feb;32(2 Pt 1):287–289.
- Ikoma A, Steinhoff M, Ständer S, et al. The neurobiology of itch. Nat Rev Neurosci. 2006 Jul;7(7):535–547.
- Johnson P, Becker L, Halpern R, et al. Real-world treatment of post-herpetic neuralgia with gabapentin or pregabalin. Clin Drug Investig. 2013 Jan;33(1):35–44.
- Li K-W, Yu YP, Zhou C, et al. Calcium channel α2δ1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J Biol Chem. 2014 Mar 7;289(10):7025–7037.
- Van Ameringen M, Patterson B, Simpson W. DSM-5 obsessive-compulsive and related disorders: Clinical implications of new criteria. Depress Anxiety. 2014 Jun;31(6):487–493.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013 Feb;31(1):31–40.
- Koo J, Lee CS. Delusions of parasitosis. A dermatologist’s guide to diagnosis and treatment. Am J Clin Dermatol. 2001;2(5):285–90.
- T-J Goon A, Yosipovitch G, Chan Y-H, et al. Clinical characteristics of generalized idiopathic pruritus in patients from a tertiary referral center in Singapore. Int J Dermatol. 2007 Oct;46(10):1023–1026.
- Reich A, Ständer S, Szepietowski JC. Pruritus in the elderly. Clin Dermatol. 2011 Feb;29(1):15–23.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: A clinical review. JAMA 2013 Dec 11;310(22):2443–2450.
- Cotes MES, Swerlick RA. Practical guidelines for the use of steroid-sparing agents in the treatment of chronic pruritus. Dermatol Ther. 2013 Apr;26(2):120–134.
- Wilson SR, Thé L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013 Oct 10;155(2):285–295.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014 Feb;133(2):448–460.
- Sabroe RA, Fiebiger E, Francis DM, Maurer D, Seed PT, Grattan CE h, et al. Classification of anti-FcepsilonRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. J Allergy Clin Immunol. 2002 Sep;110(3):492–499.
- Maurer M, Rosén K, Hsieh H-J, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013 Mar 7;368(10):924–935.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014 Mar;70(3):512–516.
- Yosipovitch G, Goon A, Wee J, et al. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000 Nov;143(5):969–973.
- Gupta MA, Gupta AK, Kirkby S, et al. Pruritus in psoriasis. A prospective study of some psychiatric and dermatologic correlates. Arch Dermatol. 1988 Jul;124(7):1052–1057.
- Bilac C, Ermertcan AT, Bilac DB, et al. The relationship between symptoms and patient characteristics among psoriasis patients. Indian J Dermatol Venereol Leprol. 2009 Oct;75(5):551.
- Reich A, Hrehorów E, Szepietowski JC. Pruritus is an important factor negatively influencing the well-being of psoriatic patients. Acta Derm Venereol. 2010 May;90(3):257–263.
- Szepietowski JC, Reich A, Wiśnicka B. Itching in patients suffering from psoriasis. Acta Dermatovenerol Croat. 2002 Dec;10(4):221–226.
- Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: Recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007 Dec;9(6):461–467.
- Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol. 2008 May;128(5):1064–1067.
- El-Baalbaki G, Razykov I, Hudson M, et al. Association of pruritus with quality of life and disability in systemic sclerosis. Arthritis Care Res. 2010 Oct;62(10):1489–1495.
- Milette K, Hudson M, Körner A, et al. Sleep disturbances in systemic sclerosis: Evidence for the role of gastrointestinal symptoms, pain and pruritus. Rheumatol Oxf Engl. 2013 Sep;52(9):1715–1720.
- Chularojanamontri L, Sethabutra P, Kulthanan K, et al. Dermatology life quality index in Thai patients with systemic sclerosis: A cross-sectional study. Indian J Dermatol Venereol Leprol. 2011 Dec;77(6):683–687.
- Goreshi R, Chock M, Foering K, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011 Dec;65(6):1107–1116.
- Razykov I, Levis B, Hudson M, et al. Prevalence and clinical correlates of pruritus in patients with systemic sclerosis: An updated analysis of 959 patients. Rheumatol Oxf Engl. 2013 Nov;52(11):2056–2061.
- Robinson ES, Feng R, Okawa J, et al. Improvement in the cutaneous disease activity of patients with dermatomyositis is associated with a better quality of life. Br J Dermatol. 2014 Jun 7.
- Shirani Z, Kucenic MJ, Carroll CL, et al. Pruritus in adult dermatomyositis. Clin Exp Dermatol. 2004 May;29(3):273–276.
- Hundley JL, Carroll CL, Lang W, et al. Cutaneous symptoms of dermatomyositis significantly impact patients’ quality of life. J Am Acad Dermatol. 2006 Feb;54(2):217–220.