An “evidence-based visit” in usual rheumatology care–beyond clinical trials and other research–appeared possible based on the Health Assessment Questionnaire (HAQ) and Arthritis Impact Measurement Scale (AIMS), which appeared in Arthritis & Rheumatism in 1980, the same year in which I moved to Vanderbilt University.1,2 I had been a junior faculty member at Stanford in 1975, as director of the Clinical Immunology Laboratory and a basic virology researcher–hardly a health services researcher. However, interactions with Jim Fries, MD, and Hal Holman, MD, fellow faculty members at Stanford and developers of the HAQ, had prepared me to recognize that quantitative, “scientific” data could emerge from usual clinical care, as well as from the laboratory.
Clinical measurement had long been a major interest, initially as a student in 1961 when I performed erythrocyte sedimentation rates (ESRs) in the Columbia University laboratory of Charles Christian, MD. This interest was expressed in 1969 in development of a radioimmunoassay for DNA antibodies, so that a diagnosis of systemic lupus erythematosus (SLE) could be based on a routine clinical test rather than a “research test.”3 Clinical decisions in rheumatoid arthritis (RA) in 1980 (and today) were based primarily on information from the patient, rather than laboratory tests, although laboratory tests were (and often remain) the only quantitative clinical information in the medical record. Self-report questionnaires could document patient history information concerning physical function and pain as quantitative scores, rather than as gestalt, nonquantitative descriptions (e.g., “ ‘doing well’ while the patient has become progressively crippled before their eyes”).4 Standard, quantitative, patient self-report data could meet criteria for “scientific” measures, similar to laboratory tests.
Theory into Practice
To incorporate quantitative clinical measurement in clinical care, I asked the Vanderbilt clinic receptionists to distribute a HAQ to patients before they saw me. This practice was implemented and maintained easily over 27 years, although the receptionists were employees of the nursing service (rather than the physicians) and turned over frequently. Review of the information provided by the patients on questionnaires was very helpful prior to performing my own history and physical examination. Leigh Callahan, PhD, Christopher Swearingen, PhD, and Raye Brooks, colleagues at Vanderbilt, helped to establish a database to provide flowsheets of quantitative self-report clinical scores, along with traditional laboratory tests and medications for all visits of all patients (with any diagnosis).
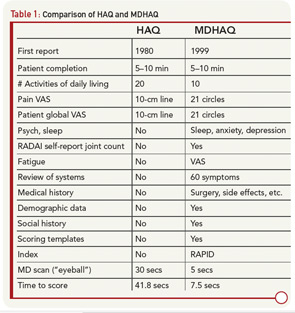
Routine completion of a HAQ in my clinic led to several changes to provide additional clinically useful information within a two-sided one-page format. The original 20 HAQ activities in eight categories were compressed to eight activities, one from each category; activities performed by all people each day were included (e.g., “shampoo your hair” was deleted). Queries concerning “aids, devices, or help from another person” were dropped, since they were usually negative and complicated scoring. A modified HAQ (MHAQ) reported in 1983 described similar results to the HAQ, though with scores lower by about 0.3 units because simplest activities were included.5 These were initial efforts in a continuous quality improvement (CQI) process that has lasted more than 25 years involving further modifications of the original two-sided, one-sheet HAQ (see Table 1), ultimately leading to a multidimensional HAQ (MDHAQ) (see Figures 1 and 2).6-8
Unexpected Observations from a Box of Records
My appreciation of the value of the evolving MDHAQ was greatly accelerated in July 1982 by results of a review of long-term outcomes of RA, which began as a summer research project by a Vanderbilt medical student, Lee Payne. William Sale, MD, an orthopedic resident there, had performed an unusually extensive evaluation, including patient questionnaires, on 75 RA patients nine years earlier in 1973, and gave me a box of their records (labeled “Jim Beam”) before he left Vanderbilt. Our investigation of the patients nine years later revealed five unexpected observations, which presented novel implications for care of patients with RA:9
- Long-term outcomes of RA were much more unfavorable than was generally recognized at the time, with severe functional declines and premature mortality rates, predicted by severity of clinical status, rather than by adverse effects of therapies.
- Treatment at that time, although described as “remission-inducing” in textbooks, was not adequate for favorable long-term results.10
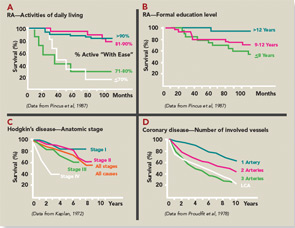
- The most significant quantitative clinical predictor of premature mortality was physical function assessed on a patient questionnaire, rather than radiographs or laboratory tests. Poor physical function scores identified a risk of less than 50% five-year survival in RA similar to stage IV Hodgkin disease or three-vessel coronary artery disease (see Figure 3). Therefore, it seemed reasonable that patient self-report questionnaire scores should be available for clinical decisions at all usual care visits.
- Formal education level was more significant in predicting premature mortality than laboratory tests or radiographs (see Figure 3), indicating the importance of socioeconomic status, patient actions, and self-management in the outcomes of RA (and all chronic diseases).11
- Results of relatively short-term clinical trials with agents such as gold salts and penicillamine did not translate into favorable long-term outcomes, indicating a need for long-term observational studies to analyze results of treatment and outcomes of RA (and all chronic diseases).
The first two observations—that RA was a severe disease with high rates of work disability and increased mortality and that the therapies available at that time were inadequate—were widely accepted by the rheumatology community; similar reports from other sites helped foster new treatment approaches. The other three observations, however, have been accepted much more slowly over the last 25 years. Patient self-report questionnaires—the most significant predictor of work disability and mortality in RA—have been introduced into only about 20% of rheumatology usual care settings, although at an accelerated rate in recent years. The importance of socioeconomic variables in health, though widely recognized far beyond RA, remains omitted from most reports of clinical trials and outcomes.11 Observational databases have been established to monitor long-term outcomes, but generally as research activities, rather than in the infrastructure of usual care. This seems unfortunate, because simple programs for self-report questionnaires allow documentation of the course of every rheumatology patient, without a need for specialized registries, although analyses of data in groups require additional expertise.
A Closer Look at Development of the MDHAQ/RAPID3
Evolution of a two-sided, one-page MDHAQ continued as one of the few questionnaires developed in actual clinical (rather than research) settings to improve care (see Table 1)—although much research data emerged.6 In 1995, it was recognized that improved patient status led to “floor effects” on the HAQ and MHAQ, i.e., scores of zero although patients nonetheless had functional limitations. Therefore, queries concerning complex activities were added—initially six and ultimately two: “walk two miles or three kilometers” and “participate in sports and recreation as you would like” (see Figure 1).7,8 Queries concerning sleep, anxiety, and depression were added, scores of which are as high or higher than for most traditional HAQ items.8 Visual analog scales (VAS) for pain and global status were converted from a 10-cm line to 21 circles (in increments of 0.5) (see Figure 1), so that a ruler is no longer required for scoring.12
Scoring templates were developed for Routine Assessment of Patient Index Data (RAPID)3, a simple 0–30 composite of three 0–10 scores for physical function, pain and patient global estimate, the 3 RA Core Data Set measures.13,14 A self-report joint count was added from the Rheumatoid Arthritis Disease Activity Index (RADAI) questionnaire.15 RADAI and the psychological status queries were scored formally in earlier versions, as RAPID4 and RAPID5, but RAPID3 scores gave similar information in much less time.13 Therefore, formal scoring of these scales is no longer advocated, though the scales provide highly useful clinical information.
The second page of the MDHAQ (see Figure 2) includes a review of systems and recent medical history. More than 25 checks for positive symptoms is characteristic of patients with fibromyalgia, some of whom may also meet criteria for diseases such as RA or SLE; this information proves quite helpful as a clue that symptoms may be due to fibromyalgia rather than inflammation, a difficult determination in some patients.16 A recent medical history summary can save at least two to three minutes for the doctor if all answers are “no,” whereas any “yes” responses should be known.17 Queries concerning change in status, exercise, a visual analog scale for fatigue, demographic measures, consent for further monitoring, and contact in the future should the patient change clinical settings also have proven their value over and over.
A two-page, four-sided “folder” MDHAQ, generally only for new patients (or those new to a database), includes a standard medical history. A Microsoft Access program provides a report in a standard medical record format. Of course, the information must be reviewed by the doctor with a further careful history and physical examination, but 75% of the medical record note is provided by patient self-report, rather than written, dictated, or typed by the doctor. A self-report questionnaire provides considerably more accurate details about most (but not all) components of the medical history than dialog with a health professional, for most (but not all) patients.
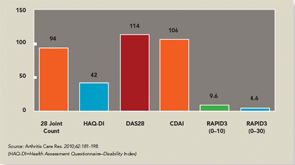
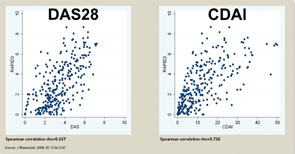
The three RA Core Data Set patient self-report scores for physical function, pain, and patient global estimate distinguish active from control treatments in clinical trials as efficiently as the three measures scored by a health professional—tender joints, swollen joints, and doctor global estimate—or laboratory tests.18-20 Therefore, RAPID3 is correlated with more traditional RA indices which include a formal joint count, Disease Activity Score (DAS) 28 and Clinical Disease Activity Index (CDAI) at levels of rho >0.6 (see Figure 5). These levels are higher than rho ~0.5 seen between ESR and C-reactive protein, indicating that RAPID3, DAS28, and CDAI address a similar construct in clinical trials and usual care settings.
RAPID3 distinguishes active from control treatments in clinical trials as efficiently as DAS28 or CDAI, reflecting the individual measures. However, RAPID3 on an MDHAQ can be scored in about five seconds, compared with 90–120 seconds for a DAS28 or CDAI (see Figure 4).21 Flowsheets of patient self-report scores, laboratory test results, and medications (see Figure 6) allow presentation of the course of a patient at a glance (which is far more useful in busy clinical settings than pages or screens of narrative information) as “evidence-based rheumatology care.”
Evidence-Based Medicine in Usual Care
The term “evidence-based medicine” has been described as “derived from many sources beyond randomized clinical trials.”22 However, a hierarchy of levels of evidence lists randomized clinical trials and meta-analyses as the highest, and observational studies at lower levels, and the term “evidence-based medicine” often is applied only to data from clinical trials.23 Of course, clinical trials provide the most rigorous data to compare the efficacy of active versus control treatments over defined periods. Nonetheless, clinical trials have many pragmatic and intrinsic limitations, such as a short time frame, patient selection, inflexible dosages, and many others.24-27
Data from patients are more significant to predict mortality than laboratory tests, radiographs, and other high-technology data.
A view of “evidence-based medicine” as based only on clinical trials reflects a “biomedical model,” the dominant paradigm of 20th century medicine, to mimic a “reductionist” laboratory experiment, isolating a single variable for a research study. A biomedical model also views information from patients as “subjective” nonquantitative narratives, in contrast to “objective” high-technology data, and mind and body as separate in health and disease. The dominance of the biomedical model in contemporary rheumatology is reflected in rapid implementation of assays for DNA antibodies in the 1970s, and anticitrullinated peptide antibodies (ACPA) in the 2000s, while most rheumatologists have been slow to collect patient self-report questionnaires.28
I hope that more health professionals will adopt ‘evidence-based’ approaches to improve patient outcomes.
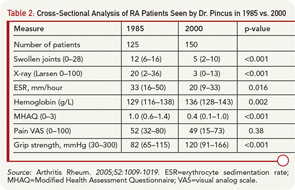
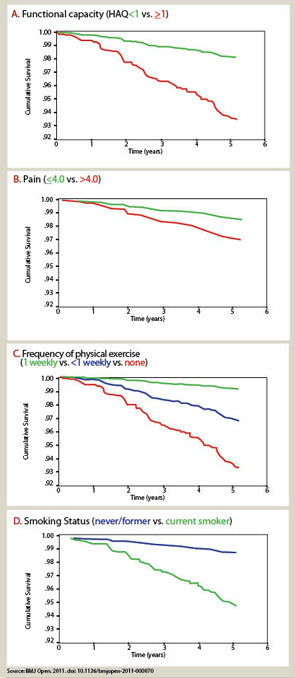
A biomedical model is most effective in acute diseases, but includes many limitations in chronic diseases, and the need for a complementary “biopsychosocial model” may be seen in studies of outcomes of RA.29 Data from patients are more significant to predict mortality than laboratory tests, radiographs, and other high-technology data. Socioeconomic status, reflecting actions of patients rather than health professionals, is highly significant in the incidence, prevalence, morbidity, and mortality of RA and many diseases, which can be explained only in small part by limited access to medical services. Much important knowledge concerning RA has been derived from observational research, not available from clinical trials, including frequent work disability and premature mortality,9 radiographic damage within the first two years,30 absence of long-term remission using traditional approaches,31 gastropathy associated with nonsteroidal antiinflammatory drugs,32 better patient status at this time compared with previous decades,33 and many others. In analyses of the effects of methotrexate, the anchor drug for RA, observational data are more accurate than meta-analyses and systematic reviews, which underestimate efficacy, effectiveness, and safety.34 Patient questionnaire data can document improved status of patients in recent years compared with earlier periods (see Table 2).33 Such questionnaire data further documented that poor functional status and limited exercise predict premature mortality in the general nondiseased population in the range of smoking and hypertension (see Figure 7).35
The strength of the biomedical model paradigm may account in large part for limited incorporation of patient self-report data concerning physical function into usual rheumatology care.28 Hypertension and diabetes were transformed to aggressive public health matters through documentation that “tight control” and “treat-to-target” of blood pressure or hemoglobin A1c level led decreased mortality rates. Similarly, it seemed important to document whether reduction of abnormal physical function scores through “treat-to-target” could reduce or even eliminate premature mortality in RA. Achieving this goal requires long-term observational studies since, ethically, clinical trials could not be conducted in a symptomatic disease such as RA over long periods (unlike asymptomatic conditions such as hypertension or hypercholesterolemia).36 However, most rheumatology reports continue to emphasize changes in radiographic and laboratory measures that have far lower significance in the prognosis of RA mortality than self-report questionnaire scores.
A few other rheumatologists have collected clinical databases since the 1980s, generally superior to mine—Fred Wolfe, MD in Wichita; Rolf Rau, MD, in Ratingen, Germany; Tore Kvien, MD, in Oslo, Norway; and Tuulikki Sokka, MD, PhD, in Jyvaskyla, Finland—to pursue “evidence-based” usual rheumatology care. I am grateful to each for their friendship and observations, which often confirmed or anteceded novel, unexpected results, indicating the value of data collection in usual care. Nonetheless, most rheumatology visits are conducted largely as they were 50 years ago when I started in rheumatology as a student—albeit many doctors are typing into an electronic medical record (EMR) instead of writing or dictating.
The Future of MDHAQ
Further developments of the MDHAQ currently in progress include: 1) an electronic version, with automated scoring and entry into a database; 2) report from the four-page MDHAQ in a standard medical history format, entirely from self-report, with no data entry or dictation or typing by the doctor; 3) methods for storage of the medical history data for both doctors and patients, which include the capacity to amend and correct stored information; and 4) integration of MDHAQ/RAPID3 into EMRs, a particularly difficult challenge in view of many incompatible systems, but one that has been solved in some settings. An MDHAQ/RAPID3 in usual care in no way prevents collecting a DAS28, CDAI, ultrasound, or any other measure and assures quantitative data at each visit.
As someone who has conducted basic laboratory investigation and supervised a clinical laboratory, I have found self-reports from questionnaires provide data that are as scientifically valid as laboratory assays, and can provide key information to facilitate patient management and improve outcome. I hope that more health professionals will adopt “evidence-based” approaches to improve patient outcomes. Readers are invited to contact me to help implement an MDHAQ/RAPID3 in their clinic settings.
Dr. Pincus is clinical professor of medicine in the division of rheumatology at New York University School of Medicine and NYU Hospital for Joint Diseases in New York City. Contact him at [email protected].
References
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137-145.
- Meenan RF, Gertman PM, Mason JH. Measuring health status in arthritis: The arthritis impact measurement scales. Arthritis Rheum. 1980;23:146-152.
- Pincus T, Schur PH, Rose JA, Decker JL, Talal N. Measurement of serum DNA-binding activity in systemic lupus erythematosus. N Engl J Med. 1969;281:701-705.
- Smith T. Questions on clinical trials (editorial). Br Med J. 1983;287:569.
- Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford health assessment questionnaire. Arthritis Rheum. 1983;26:1346-1353.
- Pincus T, Maclean R, Yazici Y, Harrington JT. Quantitative measurement of patient status in the regular care of patients with rheumatic diseases over 25 years as a continuous quality improvement activity, rather than traditional research. Clin Exp Rheumatol. 2007;25(6 Suppl 47):S69-S81.
- Pincus T, Swearingen C, Wolfe F. Toward a multidimensional health assessment questionnaire (MDHAQ): Assessment of advanced activities of daily living and psychological status in the patient friendly health assessment questionnaire format. Arthritis Rheum. 1999;42:2220-2230.
- Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a multidimensional health assessment questionnaire for standard care of patients with rheumatic diseases. J Rheumatol. 2005;32:1432-1439.
- Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864-872.
- Kelley WN, Harris ED, Jr., Ruddy S, Sledge CB. Textbook of Rheumatology. 2nd ed. Philadelphia: W.B. Saunders; 1985.
- Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Ann Intern Med. 1998;129:406-411.
- Pincus T, Bergman M, Sokka T, Roth J, Swearingen C, Yazici Y. Visual analog scales in formats other than a 10 centimeter horizontal line to assess pain and other clinical data. J Rheumatol. 2008;35:1550-1558.
- Pincus T, Bergman MJ, Yazici Y, Hines P, Raghupathi K, Maclean R. An index of only patient-reported outcome measures, routine assessment of patient index data 3 (RAPID3), in two abatacept clinical trials: Similar results to disease activity score (DAS28) and other RAPID indices that include physician-reported measures. Rheumatology (Oxford). 2008;47:345-349.
- Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (routine assessment of patient index data 3), a rheumatoid arthritis index without formal joint counts for routine care: Proposed severity categories compared to DAS and CDAI categories. J Rheumatol. 2008;35:2136-2147.
- Stucki G, Liang MH, Stucki S, Brühlmann P, Michel BA. A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Arthritis Rheum. 1995;38:795-798.
- DeWalt DA, Reed GW, Pincus T. Further clues to recognition of patients with fibromyalgia from a simple 2-page patient multidimensional health assessment questionnaire (MDHAQ). Clin Exp Rheumatol. 2004;22:453-461.
- Pincus T, Mandelin AM, Swearingen CJ. Flowsheets that include MDHAQ physical function, pain, global, and RAPID3 scores, laboratory tests, and medications to monitor patients with all rheumatic diseases: An electronic database for an electronic medical record. Rheum Dis Clin North Am. 2009;35:829-842.
- Pincus T, Amara I, Segurado OG, Bergman M, Koch GG. Relative efficiencies of physician/assessor global estimates and patient questionnaire measures are similar to or greater than joint counts to distinguish adalimumab from control treatments in rheumatoid arthritis clinical trials. J Rheumatol. 2008;35:201-205.
- Tugwell P, Wells G, Strand V, et al. Clinical improvement as reflected in measures of function and health-related quality of life following treatment with leflunomide compared with methotrexate in patients with rheumatoid arthritis: Sensitivity and relative efficiency to detect a treatment effect in a twelve-month, placebo-controlled trial. Arthritis Rheum. 2000;43:506-514.
- Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Ann Rheum Dis. 2008;67:260-265.
- Pincus T, Swearingen CJ, Bergman MJ, et al. RAPID3 on an MDHAQ is correlated significantly with activity levels of DAS28 and CDAI, but scored in 5 versus more than 90 seconds. Arthritis Care Res. 2010;62:181-189.
- Sackett DL, Rosenberg WM, Gray JM, Haynes RB, Richardson WS. Evidence based medicine: What it is and what it isn’t. It’s about integrating individual clinical expertise and the best external evidence. Br Med J. 1996;312:71-72.
- Preventive Services Task Force. Guide to clinical preventive services: Report of the U.S. Preventive Services Task Force. 2nd ed. Baltimore: Williams & Wilkins; 1996.
- Feinstein AR. An additional basic science for clinical medicine: II. The limitations of randomized trials. Ann Intern Med. 1983;99:544-550.
- Pincus T. Rheumatoid arthritis: Disappointing long-term outcomes despite successful short-term clinical trials. J Clin Epidemiol. 1988;41:1037-1041.
- Pincus T. Limitations of randomized controlled clinical trials to recognize possible advantages of combination therapies in rheumatic diseases. Semin Arthritis Rheum. 1993;23(Suppl 1):2-10.
- Pincus T, Stein CM. Why randomized controlled clinical trials do not depict accurately long-term outcomes in rheumatoid arthritis: Some explanations and suggestions for future studies. Clin Exp Rheumatol. 1997;15(Suppl.17):S27-S38.
- Pincus T, Yazici Y, Bergman MJ. Quantitative clinical rheumatology: Why is a test for anti-CCP antibodies included in most routine care for rheumatoid arthritis while a HAQ/MDHAQ remains largely a research tool? J Rheumatol. 2009;36:1563-1564.
- Thomas JW, Zenowich E, Beckwith M, Nell LJ. Genetic origin of human anti-insulin antibodies. Human Immunol. 1989;26:333-343.
- Fuchs HA, Kaye JJ, Callahan LF, Nance EP, Pincus T. Evidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol. 1989;16:585-591.
- Wolfe F, Hawley DJ. Remission in rheumatoid arthritis. J Rheumatol. 1985;12:245-252.
- Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med. 1988;109:359-363.
- Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum. 2005; 52:1009-1019.
- Pincus T, Furer V, Sokka T. Underestimation of the efficacy, effectiveness, tolerability, and safety of weekly low-dose methotrexate in information presented to physicians and patients. Clin Exp Rheumatol. 2010; 28(5 Suppl 61):S68-S79.
- Sokka T, Pincus T. Poor physical function, pain and limited exercise: Risk factors for premature mortality in the range of smoking or hypertension, identified on a simple patient self-report questionnaire for usual care. BMJ Open. 2011; Published: 18 June 2011 doi:10.1136/bmjopen-2011-000070.
- Stein CM, Pincus T. Placebo-controlled studies in rheumatoid arthritis: Ethical issues. Lancet. 1999; 353:400-403.