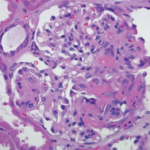
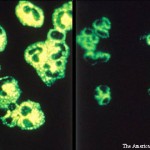
It is an exciting time in the world of vasculitis research. More clinical studies and trials are being conducted now than at any time in history. In the past ten years, four drugs have been approved by the U.S. Food & Drug Administration (FDA) and other regulatory agencies specifically for the treatment of vasculitis: Rituximab was approved for the treatment of vasculitis associated with anti-neutophil cytoplasmic antibodies (ANCA) in 2011 and was the first drug ever approved in the U.S. for any form of vasculitis. This was followed in 2017 by the approval of tocilizumab for giant cell arteritis (GCA) and mepolizumab for eosinophilic granulomatosis with polyangiitis (EGPA). In 2019, apremilast was approved for the treatment of oral ulcers in Behçet’s disease.
This progress is the direct result of international collaborations among researchers and partnerships with industry. Vasculitis research networks and patient advocacy groups have played a major role in bringing together investigators from all over the world and developing a framework for research.
Vasculitis Research Networks
The vasculitides comprise rare, complex, multisystem diseases. Clinical research in vasculitis demands multidisciplinary expertise and multicenter collaboration. Research networks have facilitated collaboration among investigators and partnerships with industry and patient advocacy groups. Conducting research under the umbrella of networks allows easy communication among collaborators, who have the advantage of working with a core team of experts who can benefit from institutional memory.
Several national and multinational vasculitis research networks have been active in clinical research. The Vasculitis Clinical Research Consortium (VCRC) is a member of the Rare Diseases Clinical Research Network, which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Office of Rare Diseases Research (ORDR), and is the largest multinational research network dedicated to clinical and translational research in all forms of vasculitis.
VCRC studies have involved all the major centers for clinical investigation in vasculitis in the U.S. and Canada, and its trials have included more than 100 sites internationally. The consortium has conducted clinical trials; implemented longitudinal cohort studies with linked biospecimens leading to many clinical, epidemiologic, biomarker development, and genetic analyses; developed and validated outcome measures in vasculitis; and established an online patient research registry (the Vasculitis Patient-Powered Research Network [VPPRN]). The VCRC and the Vasculitis Foundation (VF) also support the training of young investigators through the VCRC-VF Vasculitis Fellowship; more than 20 VCRC-VF fellows have completed the program and now lead new vasculitis centers and conduct research in the field.
The European Vasculitis Society (EUVAS) is a multinational research network dedicated to research in vasculitis; it has a particular focus on clinical trials in ANCA-associated vasculitis. The VCRC and EUVAS have collaborated on many clinical projects, including several large, recently completed and currently active trials (see Table 1, below).
Table 1: CURRENT CLINICAL TRIALS IN VASCULITIS
| TRIAL NAME RESEARCH NETWORK | BRIEF DESCRIPTION | CURRENT STATUS/ LOCATION OF SITES | CLINICALTRIALS.GOV IDENTIFIER | |
|---|---|---|---|---|
| GIANT CELL ARTERITIS (GCA) | ||||
| Investigator Initiated | ABAGART VCRC | Phase 3 placebo-controlled RCT to determine safety and efficacy of abatacept to treat GCA | Will launch in early 2021, International | NCT04474847 |
| METOGIA, FVSG | RCT to compare tocilizumab to methotrexate to treat GCA | Recruiting, France | NCT03892785 | |
| Industry Sponsored | SELECT-GCA | Phase 3 placebo-controlled RCT to determine safety and efficacy of upadacitinib to treat GCA | Recruiting, International | NCT03725202 |
| CAIN-457 | Phase 3 placebo-controlled RCT to determine safety and efficacy of secukinumab to treat GCA | Will start recruitment in May 2021, International | Pending | |
| Phase 2 placebo-controlled RCT to determine safety and efficacy of mavrilimumab to treat GCA | Completed, results presented at ACR Convergence 2020 | NCT03827018 | ||
| Investigator Initiated | RCT comparing methotrexate, mycophenolate and glucocorticoids vs. cyclophosphamide and glucocorticoids followed by azathioprine and glucocorticoids | Recruiting, China | NCT03096275 | |
| Phase 3 trial to determine safety and efficacy of leflunomide in TAK | Planning | Pending | ||
| ANCA-ASSOCIATED VASCULITIS (AAV): GRANULOMATOSIS with POLYANGIITIS (GPA) and/or MICROSOPIC POLYANGIITIS (MPA) | ||||
| Investigator Initiated | RITAZAREM VCRC-EUVAS | Phase 3 RCT to compare rituximab to azathioprine to treat relapsing AAV | International, completed, final results presented at ACR Convergence 2020 | NCT01697267 |
| ABROGATE VCRC-EUVAS | Phase 3 placebo-controlled RCT to determine efficacy and safety of abatacept to treat relapsing, mild-moderate GPA | Recruiting, International | NCT02108860 | |
| TAPIR VCRC | RCT to compare maintenance with prednisone 5 mg daily for 6 months vs. discontinuation of prednisone in AAV | Recruiting, North America | NCT01933724 | |
| MAINEPSAN FVSG | RCT to compare maintenance with prednisone 5 mg daily for 12 months vs. discontinuation of prednisone in AAV | Recruiting, France | NCT03290456 | |
| RituxGoPro FVSG | RCT to determine efficacy of rituximab/glucocorticoids vs. glucocorticoids alone to treat non-severe MPA | Recruiting, France | NCT03920722 | |
| SATELITE FVSG | RCT to compare tocilizumab vs. abatacept vs. rituximab+cDMARD in treatment of refractory GPA | Recruitment will start in 2021, France | pending | |
| LEFAZAREM | RCT comparing leflunomide vs azathioprine for maintenance of remission in AAV | Recruitment will start in 2021, China | pending | |
| RCT comparing efficacy and safety of IV tocilizumab vs. IV cyclophosphamide in active AAV | Recruiting, Japan | |||
| Industry Sponsored | ADVOCATE | Phase 3 placebo-controlled RCT to compare avacopan with no glucocorticoids vs. glucocorticoids, both in addition to rituximab or cyclophosphamide, for inducing and sustaining remission of AAV | Completed, International | NCT02994927 |
| IXchange | Phase 2 RCT to investigate safety and efficacy of IFX-1. Part 1: Treatment with IFX-1 with varying doses of glucocorticoids to induce remission in AAV. Part 2: Treatment with IFX-1 alone vs. IFX-1 with glucocorticoids | Part 1: completed Part 2: recruiting, Europe | NCT03895801 | |
| IXplore | Phase 2 RCT to investigate safety and efficacy of two different dose regimens of IFX-1 to induce remission in AAV | Recruitment completed, North America | NCT03712345 | |
| ANCA-ASSOCIATED VASCULITIS: EOSINOPHILIC GRANULOMATOSIS with POLYANGIITIS (EGPA) | ||||
| Investigator Initiated | REOVAS FVSG | RCT to compare rituximab to conventional treatment to induce remission of EGPA | Study completed, results in 2021, France | NCT02807103 |
| MAINRITSEG FVSG | RCT to compare rituximab to azathioprine to maintain remission of EGPA | Recruiting, France | NCT03164473 | |
| EMERGE FVSG | RCT to compare mepolizumab to conventional treatment to induce remission of EGPA | Starting January 2021, France | Pending | |
| Industry Sponsored | MANDARA | Phase 3 RCT to compare the safety and efficacy of benralizumab vs. mepolizumab to treat EGPA | Recruiting, International | NCT04157348 |
| BEHÇET’S DISEASE | ||||
| Industry Sponsored | Phase 2 placebo-controlled RCT to determine safety and efficacy of pentoxyfylline gel to treat oral ulcers in Behçet’s disease | Recruiting, Turkey | NCT03888846 | |
| EOSINOPHILIC GRANULOMATOSIS with POLYANGIITIS, GIANT CELL ARTERITIS, GRANULOMATOSIS with POLYANGIITIS, MICROSCOPIC POLYANGIITIS, POLYARTERITIS NODOSOA or TAKAYASU’S ARTERITIS | ||||
| LoDoNaVasc VCRC | RCT comparing low-dose naltrexone vs. placebo for treatment of self-reported physical health | Recruiting, North America | NCT03482479 | |
| CUTANEOUS VASCULITIS | ||||
| ARAMIS VCRC | RCT comparing the efficacy of 3 drugs (azathioprine, colchicine and dapsone) in isolated cutaneous vasculitis | Recruiting, International | NCT02939573 | |
VCRC: Vasculitis Clinical Research Consortium; EUVAS: European Vasculitis Study Group; FVSG: French Vasculitis Study Group; VF: Vasculitis Foundation; RCT: randomized controlled trial; ANCA: anti-neutrophil cytoplasmic antibody; cDMARD: conventional disease-modifying anti-rheumatic drug; EGPA: eosinophilic granulomatosis with polyangiitis; GCA: giant cell arteritis; GPA: granulomatosis with polyangiitis; MPA: microscopic polyangiitis.
Several countries have national vasculitis research networks. The French Vasculitis Study Group (FVSG) has been particularly successful in conducting multicenter clinical trials, with a large number of centers in France participating in the work. Similar research networks in Canada, Japan and Turkey have conducted national studies and collaborated on multinational projects.
For many years, investigators from different parts of the world have met annually at the Vasculitis Investigators Meeting, hosted by the VCRC. At this major venue, investigators, representatives from industries, research networks and patient advocacy groups discuss the current state of clinical research in different types of vasculitis. This meeting also creates a platform to initiate novel projects and develop new collaborations. Over time, the meeting has experienced a significant increase in the number of participants and research projects presented, serving as a testament to the success of multinational collaboration.
Patient Advocacy Groups
In addition to research networks, patient advocacy groups have significantly contributed to clinical research in vasculitis. The VF, the world’s largest umbrella advocacy group for patients with vasculitis, has had a longstanding partnership with the VCRC in research and training, including through the VCRC-VF Fellowship and the VPPRN.
In the VPPRN, patients with vasculitis self-enroll, provide detailed clinical information and volunteer to take part in research studies online. This partnership has enabled direct engagement of patients, caregivers, investigators and health systems to develop research methods to electronically collect health records and patient-reported data on a large number of patients with various forms
of vasculitis.
More than 3,000 patients are enrolled in the VPPRN, which has completed, and has ongoing, projects in multiple forms of vasculitis. For example, the Vasculitis Pregnancy Registry (V-PREG) is an ongoing initiative studying the experience of women with vasculitis who become pregnant and the outcomes of their pregnancies.
The strength of this ready-for-research population was demonstrated by the VPPRN’s ability to rapidly launch a longitudinal study to understand how patients with vasculitis were dealing with the COVID-19 pandemic.
Several other national and disease-specific patient advocacy groups are also active around the world, each providing support and education to patients with vasculitis and often partnering with academic centers to conduct research.
Registries & More
Vasculitis research networks have developed longitudinal registries for different types of vasculitis by connecting major academic centers internationally. For rare diseases, such as vasculitis, longitudinal registries have been the foundation of clinical studies, creating large and unique databases of comprehensive clinical information, sometimes linked to biologic materials stored at centralized repositories.
For example, the VCRC Vasculitis Biorepository contains a large collection of specimens (i.e., DNA, serum, plasma, urine and tissue) from patients with any of several forms of vasculitis stored centrally at the University of Pennsylvania. All samples are linked to comprehensive clinical and phenotypic data.
Longitudinal clinical and biospecimen repositories exist for granulomatosis with polyangiitis (GPA), EPGA, microscopic polyangiitis (MPA), GCA, Takayasu’s arteritis (TAK) and polyarteritis nodosa (PAN). The VCRC has plans to start longitudinal registries for primary central nervous system vasculitis (CNSV) and relapsing polychondritis (RP), which will enable systematic collection of biologic samples, clinical data imaging and tissue, as well as the development of consensus-based diagnostic and classification criteria. Data from longitudinal registries have been used for many epidemiological studies, biomarker development and genetic analyses.
Other ongoing clinical and translational projects worldwide include a project of the FVSG, PNEUMOVAS (ClinicalTrials.gov identifier: NCT02463578), testing the immunogenicity and safety of two innovative anti-pneumococcal vaccine strategies against standard vaccination regimen after induction of remission of ANCA-associated vasculitis with rituximab; a VCRC multicenter observational study to evaluate histopathology and transcriptomics of cutaneous lesions in systemic vasculitis (CUTIS; ClinicalTrials.gov Identifier: NCT03004326); and an ongoing study of interstitial lung disease in ANCA-associated vasculitis in Japan.
Several successful genome-wide association studies have been made possible through the combined efforts of VCRC and EUVAS, as well as Japanese and Turkish vasculitis groups. The ongoing VCRC Genetic Repository collects DNA and clinical data from patients with any of several vasculitides. In addition to large genome-wide studies, candidate gene studies are regularly conducted in vasculitis, including a recent analysis of DADA2 mutations in patients with PAN in collaboration with the NIH.
Outcome Measures & Classification Criteria
Successes of clinical trials in vasculitis were greatly advanced by projects focused on development, validation and acceptance by the international research community of standardized outcome measures. The Outcome Measures in Rheumatology (OMERACT) Vasculitis Working Group has helped advance much of this work (see Table 2), including in ANCA-associated vasculitis, large-vessel vasculitis and Behçet’s disease. OMERACT is an international collaboration of investigators, clinicians, biopharmaceutical industry professionals and patient partners involved in outcome measures in rheumatology.
Table 2: OMERACT Outcome Measures in Vasculitis Projects
| PROJECTS | CURRENT STATUS | References |
|---|---|---|
| ANCA-ASSOCIATED VASCULITIS | ||
| Core set of outcome measures for use in trials | • Core set of domain and instruments endorsed and in widespread use | Merkel PA et al. J Rheumatol 2011 |
| Disease-specific patient-reported outcomes | • The AAV-PRO instrument developed and validated • Now in use in clinical trials and other projects • Translations underway in many different languages | Robson JC et al. Ann Rheum Dis. 2018 |
| Treatment response criteria | • American College of Rheumatology-European League Against Rheumatism-supported project to develop data-driven response criteria in ANCA-associated vasculitis • Systematic literature review of outcome measures in randomized trials revealed substantial heterogeneity and non-inclusion of important outcome measures • Ongoing international physician and patient Delphi exercise to select items for inclusion in treatment response criteria • Planned process to lead to an expert panel finalizing response criteria, followed by developing of a weighting system | Monti S et al. Arthritis Rheumatol. 2019 Monti S et al. Semin Arthritis Rheum. 2020 |
| LARGE-VESSEL VASCULITIS | ||
| Core set of outcome measures for use in trials | • Systematic literature review completed • International Physician Delphi exercise and qualitative patient interviews identified domains of interest and outcome measures • Some common and some disease-specific measures for giant cell arteritis and Takayasu’s arteritis identified • Preliminary core set of domains for trial in large-vessel vasculitis published | Direnskeneli H. et al. J Rheumatol. 2011; Aydin SZ et al. Curr Opin Rheumatol. 2015; Sreih AG et al. J Rheumatol. 2017; Aydin SZ et al. J Rheumatol. 2019 |
| Patient preferences and patient-reported outcomes | • Qualitative research in multiple countries conducted with patients with giant cell arteritis or Takayasu’s arteritis led to understanding of themes central to any future patient-reported outcome instrument • Next steps: • Development of candidate questionnaire items for disease-specific patient-reported outcome instrument for giant cell arteritis and Takayasu’s arteritis | Robson J et al. Ann Rheum Dis. 2018 Tomasson G et al. Arthritis Rheumatol. 2016 Sreih AG et al. Clin Exp Rheumatol. 2018 |
| Development of definitions of disease states and response criteria for use in clinical trials | • Define disease states and definitions of flare for giant cell arteritis and Takayasu’s arteritis • Next steps: • International patient Delphi to complement completed physician Delphi • Expert meeting including patient partners for item reduction and drafting data elements for study • Prospective data collection: multicenter, international study to collect data using items generated in previous steps and define active disease, remission, low disease activity • Develop response criteria in giant cell arteritis for use in clinical trials and other research studies • Next steps: • International Steering Committee formed with full study plans for a systematic literature review, Delphi exercise, and prospective data collection to subsequently generate the response criteria | Aydin SZ et al. J Rheumatol. 2017 |
| BEHÇET’S DISEASE | ||
| Core set of outcome measures for use in trials | • Core set of domains of illness for inclusion in all clinical trials in Behçet’s disease developed and endorsed • Next steps: • Identification or development of validated outcome measurement tools for each domain in core set | Hatemi G et al. Semin Arthritis Rheum. 2021 |
Another landmark research project in vasculitis conducted in the past 10 years is the Diagnostic and Classification Criteria for Vasculitis (DCVAS) project (ClinicalTrials.gov identifier: NCT01066208), funded by the ACR, the European League Against Rheumatism (EULAR), the VF and other sources. DCVAS is a multinational, observational study designed to update classification criteria and develop diagnostic criteria in vasculitis. The largest vasculitis research project in the world, DCVAS involves comprehensive data collection on thousands of patients at more than 100 sites across 30 countries in Asia, Australia, Europe, North America and South America.
Classification criteria in vasculitis were originally developed 30 years ago, before the advent of ANCA testing and newer imaging modalities. It was important to develop new classification criteria for successful and accurate recruitment into clinical trials. Draft classification criteria for the three types of ANCA-associated vasculitis, Takayasu’s arteritis and GCA were presented at ACR and EULAR annual meetings in 2019, and the final versions are expected to be published soon.
The DCVAS group is also considering approaches to establishing diagnostic criteria. Additionally, the large DCVAS database has been used by investigators for the conduct of clinical and epidemiologic studies, and remains an important research resource.
Clinical Trials
Tremendous progress has been made in the field of clinical trials in vasculitis. Several investigator-initiated, industry-sponsored and combined investigator-industry hybrid trials are ongoing. Current approaches to the treatment of ANCA-associated vasculitis have significantly improved survival and other outcomes.
Current regimens are often associated with serious side effects, such as infections and other complications of high-dose and prolonged glucocorticoid use. Trials involving new glucocorticoid-sparing induction regimens using novel complement component 5a inhibitors (i.e., avacopan and IFX-1) have been conducted or are underway.
Several ongoing trials in North America, Europe and Japan are also testing low-dose and shorter duration of glucocorticoids for the maintenance of remission in ANCA-associated vasculitis (see Table 1). Following the success of trials of rituximab as a treatment for ANCA-associated vasculitis, additional trials tested this drug for new indications, such as relapsing ANCA-associated vasculitis, mild to moderate MPA and EGPA. Other drugs and treatments are also under investigation for GPA, EGPA and MPA.
Clinical trials in large-vessel vasculitis have been less frequently conducted than for ANCA-associated vasculitis, but are now seeing increasing success. There was no FDA-approved drug for GCA until 2017. The success of the GiACTA trial, which led to the approval of tocilizumab for GCA, generated further interest in this disease. Presently, several national and international clinical trials in GCA are testing different biologics and small molecule inhibitors. Two clinical trials are ongoing in Takayasu’s arteritis, an extremely rare type of vasculitis, in Turkey and China (see Table 1).
Exciting advancements have been made through trials in other vasculitides, such as Behçet’s disease and cutaneous vasculitis, some of which incorporate patient-reported physical health as the primary outcome measure (ClinicalTrials.gov identifier: NCT03482479).
Despite the substantial success of clinical trials in several vasculitides, there is still a relative lack of clinical trials and approved drugs in many types of vasculitis, such as PAN and urticarial vasculitis. The relative rarity and heterogeneity in clinical presentations are among the several challenges in studying these diseases. However, with the growth and sophistication of international collaborations and networks, these challenges are expected to be overcome.
Summary
The past 25 years have witnessed extraordinary advancement in the clinical research in vasculitis, with the successful completion of many clinical trials that have led to marked changes in the standard of care, drug approval by regulatory agencies and improved outcomes for patients. The successes of these trials are a direct result of several factors, especially the development and use of standardized disease definitions and outcome measures, the formation of research networks to foster international collaboration, effective partnerships with the biopharmaceutical industry and patient advocacy groups.
Further exemplifying success in the field, the first-ever set of ACR/Vasculitis Foundation treatment guidelines for different vasculitides were developed. These guidelines were presented at the 2019 ACR/ARP Annual Meeting, with plans for publication in 2021. These guidelines were strongly influenced by the clinical trials conducted in the past two decades and epitomize the tremendous progress made in this field.
 Shubhasree Banerjee, MD, is an assistant professor of clinical medicine, Penn Vasculitis Center, Division of Rheumatology, Department of Medicine, University of Pennsylvania, Philadelphia. Contact her at [email protected].
Shubhasree Banerjee, MD, is an assistant professor of clinical medicine, Penn Vasculitis Center, Division of Rheumatology, Department of Medicine, University of Pennsylvania, Philadelphia. Contact her at [email protected].
 Peter A. Merkel, MD, MPH, is a professor of medicine and epidemiology, Penn Vasculitis Center, Division of Rheumatology, Department of Medicine, Division of Clinical Epidemiology, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania. Contact him at [email protected].
Peter A. Merkel, MD, MPH, is a professor of medicine and epidemiology, Penn Vasculitis Center, Division of Rheumatology, Department of Medicine, Division of Clinical Epidemiology, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania. Contact him at [email protected].
Disclosures
Dr. Merkel reports receiving funds for the following activities in the past two years:
- Consulting: AbbVie, AstraZeneca, Biogen, Boehringer-Ingelheim, Bristol Myers Squibb, Celgene, ChemoCentryx, CSL Behring, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Insmed, Janssen, Kiniksa, Magenta, Pfizer, Sparrow and Talaris.
- Research: AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Celgene, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline and InflaRx.
- Royalties: UpToDate.