Credit: Adobe Stock | Trsakaoe
BALTIMORE—For most patients, the onset of systemic sclerosis (SSc) is heralded by new or worsening Raynaud’s phenomenon. However, the complications of the disease thereafter can be varied and severe.
At the 20th Annual Advances in the Diagnosis and Treatment of the Rheumatic Diseases Symposium at Johns Hopkins School of Medicine, Baltimore, Laura Hummers, MD, ScM, associate professor of medicine, co-director of the Scleroderma Center and clinical director for the Division of Rheumatology, Johns Hopkins School of Medicine, treated the audience to important clinical pearls in her presentation, Update on Scleroderma.
Evolution of SSc
At the start of the lecture, Dr. Hummers explained that our understanding of SSc has evolved in recent decades, and even the nomenclature used for the condition reflects this more nuanced view of the disease.
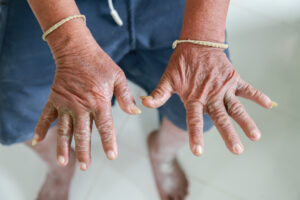
Example: Clinicians used to label some patients as having CREST, an acronym that stands for calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly and telangiectasia. Dr. Hummers advises that rheumatologists avoid this terminology because it implies these features are only seen in limited cutaneous disease when, in fact, they may appear in patients with diffuse cutaneous disease as well.
In 2013, the ACR and EULAR published classification criteria for SSc in which a score of 9 or more would indicate definite SSc. The criteria include puffy hands, sclerodactyly, digital pits or ulcers, telangiectasias, abnormal nailfold capillaries, pulmonary arterial hypertension and/or interstitial lung disease (ILD), Raynaud’s phenomenon and SSc-specific autoantibodies (i.e., anticentromere, anti-topoisomerase and anti-RNA polymerase III). Dr. Hummers pointed out that skin thickening of the fingers of both hands extending proximal to the metacarpophalangeal joints would be sufficient for classification as definite SSc.1
Pulmonary Hypertension
Regarding pulmonary hypertension, Dr. Hummers explained that this manifestation of disease can be insidious and develop over the course of many years. Pulmonary arterial hypertension (PAH) is the most common form of pulmonary hypertension in patients with SSc, but it can also be due to ILD, pulmonary veno-occlusive disease, or left ventricular systolic or diastolic dysfunction with increased left atrial pressure. All patients with scleroderma should be screened at least annually with transthoracic echocardiogram and pulmonary function testing. Risk factors/predictors of PAH in SSc include anticentromere antibodies, an age of onset above 60 years, severe Raynaud’s phenomenon, low diffusing capacity—especially relative to forced vital capacity—and elevated or rising right ventricular systolic pressure (RVSP) on echocardiogram.
