Luiscar74/shutterstock.com
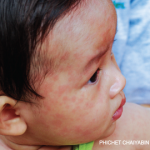
CHICAGO—In October of last year, a 52-year-old woman came to see Kevin Winthrop, MD, MPH, associate professor in infectious diseases, public health and preventive medicine at the Oregon Health & Sciences University. She had rheumatoid arthritis and was taking methotrexate and prednisone. She had had little change in her RA disease severity and was considering starting on a biologic therapy.
She had received a Bacillus Calmette-Guérin (BCG) vaccine—a vaccine primarily used against tuberculosis—as a child in Switzerland, although she now lived in the U.S. She had had shingles six months earlier. Dr. Winthrop brought up the idea of vaccination. The patient rolled out a litany of objections—that she had an egg allergy, that her husband gets the flu from his shots, that vaccines have mercury in them that causes Alzheimer’s disease.
Dr. Winthrop explained that all of those concerns were unfounded, and she became willing to receive one vaccine that day. So which one to give?
There is no clear right answer—flu, Pneumovax 23 (pneumococcal vaccine polyvalent) for Pneumococcus or even a shingles vaccine are options—but Dr. Winthrop suggested that the intramuscular flu shot might be wise, given the time of year.
When to Vaccinate
The issue of vaccinating patients with rheumatic diseases often leaves physicians in a gray zone: Patients need to be protected against diseases to which they can be more susceptible, but the vaccine itself can carry more risk in these patients. So doctors have to weigh questions of timing, benefit and risk, Dr. Winthrop said at the ACR’s State-of-the-Art Clinical Symposium. He touched on data that can help give guidance and on the future direction of vaccinations in these patients.

Dr. Winthrop
“We have very little data on how your DMARDs affect the effectiveness of vaccines,” Dr. Winthrop said. “But we do have data on immunogenicity, and we presume that less immunogenicity equals less in protection.”
There is evidence that methotrexate “severely diminishes” the response to Pneumovax and that rituximab can essentially wipe out the response to the flu vaccine and Pneumovax, he said. So those vaccines should not be given near the time rituximab is given.
There are also some data showing that abatacept can lower the responses of these vaccines.
But the evidence suggests that TNF-blockers and tocilizumab “don’t really change the responses that much,” he said. The JAK-inhibitor tofacitinib can decrease the response to Pneumovax about as much as methotrexate does, he said.1
The vaccines to be most aware of, because of their potential risks, are both the nasal and the intramuscular flu vaccines, shingles, hepatitis B, pneumococcal and tetanus-diphtheria-pertussis.
The conjugate pneumococcal vaccine PCV-13 is more immunogenic than Pneumovax, but how to use it in adults is a subject of debate. “It’s still a moving target,” Dr. Winthrop said. In a pivotal trial out of the Netherlands last year—in a population that was vaccine naive because no pneumococcal vaccine is routinely given in the Netherlands—the vaccine had 45.6% efficacy against vaccine-type community-acquired pneumonia.2
For those with immunocompromising conditions, such as RA, the Advisory Committee on Immunization Practices recommends giving PCV-13 followed by Pneumovax to those who have not previously received Pneumovax; giving PCV-13 to those who already have received one dose of Pneumovax, after waiting at least a year between the vaccines; and, for those already having received two doses of Pneumovax, to give PCV-13 if a year has passed since the last Pneumovax vaccination, with those under 65 eligible for one additional Pneumovax after 65.
For those over 65, a high-dose Fluzone vaccine might be called for. A 2014 study found it produced a higher level of protective titers, with similar adverse events, than the standard dose.3
For those on rituximab, a delay in flu vaccine is called for, studies suggest, because a much higher rise in titers has been seen among those who wait a longer period.
Dr. Winthrop added that he tends to wait until later in the flu season, when the risk of infection is higher epidemiologically, to give the flu shot, as long as he expects to see the patient in time to give them the shot.
“I don’t vaccinate my patients in September, I don’t even vaccinate them in October,” he said. “I vaccinate them later in the year, if I can. If I’m not going to see them again for six months or eight months, then that’s your opportunity to vaccinate, and you should do it in October. But if you know your flu epidemiology, you may want to wait.”
Herpes Zoster
As for shingles, Dr. Winthrop referred back to his 52-year-old patient, who raised a vexing timing question. She had gotten shingles six months earlier.
“I don’t know how long you need to wait before it’s really worth giving the vaccine again—there’s no right answer,” he said. But he did offer that a zoster vaccine expert, Michael Oxman of the University of California San Diego, told him that he would wait a year. So that is what Dr. Winthrop does in these cases.
The vaccine is important for rheumatic patients, because the rate of shingles is markedly higher for those with lupus, inflammatory bowel disease, RA and psoriatic arthritis.
The ACR recommends the herpes zoster vaccine for those 50 and up, with methotrexate, prednisone and azathioprine not contraindicated, but with the contraindications of biologics and tofacitinib.
There is evidence that methotrexate ‘severely diminishes’ the response to Pneumovax ….
Dr. Winthrop suggested that among some patients, even earlier vaccination should be explored because—at least among lupus patients—people as young as 21 to 30 years old have been found to have higher incidence rates of herpes zoster.
“I do think it raises the question as to whether we should be vaccinating younger people with this vaccine in some of these disease groups, but that’s something that needs to be tested,” he said.
The safety and efficacy of Zostavax in RA is still being studied, including with a trial of patients on biologics and TNF-blockers that he is overseeing. He has reason to think the vaccines are likely safe, he said. Medicare data show that mistaken vaccinations in 550 of such patients produced no ill effects after about 40 days.
A herpes zoster vaccine that is not live is under development, he said, which could bring a valuable new option for physicians. But Dr. Winthrop added that it’s an adjuvenated vaccine with very high efficacy rates—up to 98%—and there is some concern that it may be too potent for patients with rheumatic diseases.
“I think it’s promising,” he said. “But I do worry that maybe it’s too adjuvenated. Maybe in your population, it will cause RA flares or PsA flares—I don’t know. We totally need to test it out in your group. And if it’s safe, then, boy, this is going to be a great thing for you. But we’ll just have to see.”
Thomas R. Collins is a freelance medical writer based in Florida.
References
- Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016 Apr;75(4):687–695.
- Isturiz R, Webber C. Prevention of adult pneumococcal pneumonia with the 13-valent pneumococcal conjugate vaccine: CAPiTA, the community-acquired pneumonia immunization trial in adults. Hum Vaccin Immunother. 2015;11(7):1825–1827.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014 Aug 14;371(7):635–645.