What has our limited clinical experience taught us about the safety profile of anti-TNF therapy in SLE? Unfortunately, this is not a simple question. Immunologically, an increase in lupus-specific autoantibodies has almost always been observed. However, we are not aware of a single lupus flare in association with such an increase at the time of anti-TNF therapy or shortly thereafter. Why flares do not occur is still unclear. The answer that we have observed too few patients probably will not hold muster anymore. The argument that anti-DNA antibodies developing during TNF may not be pathogenic appears implausible given that the antibodies are high affinity (Farr assay)
(CLIFT) antibodies to double-stranded DNA and occurred in patients who obviously made pathogenic antibodies before the TNF blockade.
One straightforward hypothesis to explain the presence of anti-DNA without nephritis is that TNF blockade protects against the negative effects of the antibodies. Another more elaborate theory invokes rapid changes in the levels or composition of immune complexes given their kinetics during and after TNF blockade. At the moment, we do not know why anti-DNA antibodies can occur without nephritis. Such a situation is not without precedent in lupus, however, and occurs with serologically active, clinically inactive lupus (i.e., high anti-DNA but no nephritis). The one immunologically mediated event seen and reported during anti-TNF therapy was deep venous thrombosis in a patient not on anticoagulation despite antiphospholipid syndrome; this event was associated with an increase in preformed (and presumably pathogenic) antiphospholipid antibodies. Thrombotic events thus may prove to be an important safety signal when TNF blockers are used for the treatment of lupus.
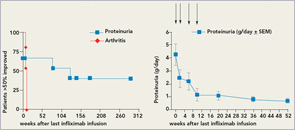
There are other unresolved issues. Infections are common and dangerous in patients with severe SLE, and experience suggests that this will also hold true under TNF blockade. Given the relatively low numbers of treated patients and potential reporting bias, numbers will not be significant to judge the impact on TNF blockade on susceptibility to infection in lupus. However, urinary tract infections during trials were quite common and might have been linked to proteinuric loss of anti-TNF antibodies. In patients under longer term TNF blockade, more serious and even one fatal infection (Legionella pneumonia) have been observed. In addition, one patient who was also on long-term anti-TNF treatment developed a cerebral lymphoma.10 Finally, after retreatment and in patients not concomitantly treated with azathioprine, mycophenolate, or methotrexate, severe infusion reactions occurred.1,12 Most of these safety issues could probably be minimized if anti-TNF therapy were restricted to induction therapy, as long-term TNF blockade in SLE appears to be less safe. Thus, a short course of induction treatment for lupus nephritis might have a more favorable risk–benefit profile than chronic treatment of lupus arthritis.
After observing the beneficial effects of TNF blockers in sick lupus nephritis patients, we believe that it would be a terrible shame if we forever lost the opportunity to study TNF blockade in a controlled clinical trial setting.
TNF Blockade and Lupus Nephritis
In lupus nephritis, recent clinical trial experience indicates the difficulty of demonstrating superiority of new therapies when compared with standard therapies. However, it is clear that there are patients for whom standard therapies are not effective. We believe that TNF blockade should primarily be tested in refractory lupus nephritis patients with high-grade proteinuria, given the available data. An induction regimen in combination with azathioprine or mycophenolate would be a reasonable option. From the infliximab experience, success was mostly achieved with high doses (5mg/kg), and occasional 3mg/kg therapy appeared insufficient. Thus, high-dose short-term therapy would be the goal. Anti-TNF therapy could either be added to mycophenolate induction in new onset lupus nephritis or used as a rescue approach in refractory lupus nephritis.
