Despite the desire of physicians to use scientific evidence to understand disease and its management, often, the experience with a single patient frames our perspective and influences our practice and research. Regarding tumor necrosis factor (TNF) blockade in systemic lupus erythematosus (SLE)—a topic of great controversy and confusion—consider the following case history and its implication for this modality of targeted therapy.
The Case
A middle-aged woman with lupus nephritis had suffered from serious disease manifestations for more than half a year. Almost 10 years prior to the recent period of intense disease activity, she had received the National Institutes of Health regimen for treatment of her lupus nephritis; she also underwent curative resection of a bladder carcinoma. Now, 10 years later, renal biopsy confirmed recurrent active class IV nephritis. Mycophenolate mofetil (MMF) was not an available option at that time. Thus, cyclophosphamide was started, and particular care was taken to protect her bladder as much as possible.
Unlike the situation with her first episode of active disease, the patient’s nephritis did not respond. Rather, her proteinuria increased to approximately 6 grams per day, and she developed nephrotic syndrome, suffering severely from edema of her legs. She also developed allergic reactions to mesna, first mild, then severe. Even though the mode of application of mesna was changed to instillation into the bladder, she suffered an anaphylactic reaction with subsequent treatment. Immunoabsorption using Therasorb immunoglobulin (Ig) G columns was the next attempt to treat her ongoing lupus nephritis. In contrast to other patients who responded to this intervention, she again had no or minimal benefit.
This experience placed her on the list for the first open-label trial of infliximab in SLE. Azathioprine was started in parallel with the infliximab in accordance with the protocol. One week after the first infusion of infliximab, her leg edema resolved completely. A few weeks later, her proteinuria fell to less than 1.5 grams per day and her serum albumin rose steadily. Despite some problems with azathioprine, which had to be stopped, she did well for more than one year. Although she encountered other problems, her good response to infliximab, along with that of other patients, laid the foundation for controlled clinical trials of anti-TNF for the treatment of lupus nephritis.1
Effects of TNF Blockade in SLE
Before contemplating the role of TNF blockade in SLE, it is important to highlight the major issues underlying our current knowledge of the role of TNF in lupus. In essence, this cytokine has two major effects: 1) TNF can downregulate the specific immune response and may also help control production of autoantibodies; and 2) TNF is a strong proinflammatory mediator with direct effects on blood vessels as well as lymphoid and myeloid cells. Both actions may play a role in the pathogenesis of human SLE and the role of TNF in mediating severe disease ma1nifestations.
The immunoregulatory role of TNF was first clearly shown in an autoimmune mouse model, specifically the F1 hybrid of New Zealand black and New Zealand white (NZB/W) mouse (see “Mouse Model,” below). Surprisingly, these mice showed low levels of TNF and had a reduction of disease manifestations when TNF was administered. These findings suggested that TNF inhibited rather than stimulated autoreactivity, a marked difference from the action of this cytokine in other disease models such as collagen-induced arthritis, where, in contrast to SLE, autoantigen may largely be accessible because of the inflammatory process. Although the paradoxical effect of TNF was primarily observed with the NZB/W mice among lupus models, these findings nevertheless raised an alarm bell about the use of TNF blockers in treating human lupus, causing caution in exploring this approach, even in patients with refractory disease.
In a clinical setting, an analogous effect occurred when patients developed antibodies to double-stranded DNA (dsDNA) and, sometimes, drug-induced lupus-like syndromes when treated with TNF blockade for rheumatoid arthritis (RA) or Crohn’s disease (see “TNF Blockade and Findings of Lupus,” p. 17).1,2 In these settings, drug-induced lupus resulting from a TNF blocker is typically mild and only rarely results in internal organ manifestations, such as lupus nephritis. Importantly, drug-induced lupus is self-limited upon cessation of TNF blocker therapy. Nevertheless, these two sets of observations, one from mouse and one from man, sparked heated debate when TNF blockade was considered as a potential treatment for SLE.
In addition to findings in murine lupus mouse models and clinical use in RA, the limited clinical experience with infliximab in SLE also suggested immunoregulatory effects of TNF that could have an impact on autoreactivity. Thus, levels of both anti-dsDNA antibodies and anti-cardiolipin antibodies increased, albeit transiently, when the first seven patients with this disease were treated in an early clinical trial of four infliximab infusions (300mg fixed dose, approximately 5mg/kg for most patients, at Weeks 0, 2, 6, and 10) in combination with azathioprine (or methotrexate).3 In fact, one patient with antiphospholipid syndrome developed venous thrombosis associated with such an increase.1 In contrast, no clinical flares have been observed by any of us or reported by any other group in SLE patients with increasing anti-dsDNA antibodies following TNF blockade.
Given the collective experience with the potent and beneficial effects of TNF blockade in diseases such as RA, psoriatic arthritis, and ankylosing spondylitis, modern-day rheumatologists are well aware that TNF is a strong inflammatory mediator in those diseases. In contrast, the role of TNF in the pathogenesis of SLE and lupus nephritis is far from clear, but the field has been beset by strong feelings, opinions, and worries arising from the existing data, which are actually quite limited.
Mouse Model
In the NZB/W F1 mice, the lupus phenotype in part depends on TNF deficiency stemming from the NZW parent.15 High-dose administration of TNF early in their life can delay disease, but long-term administration does not prevent lupus.16 These findings were more recently reiterated in TNF-deficient NZB mice, and most recently in TNF-receptor double-deficient NZB/W mice, all of which also developed severe disease.17,18
To Move Ahead
To try to make headway in this field, it is important to scrutinize the scientific evidence in this regard. TNF is clearly overexpressed in SLE. High levels of TNF have been found in SLE sera, where they closely correlate with SLE disease activity.4 Despite a concomitant increase in soluble TNF receptors, the increased TNF is bioactive.5 Furthermore, at the level of inflamed tissue, TNF expression has been found in lupus skin lesions and in inflamed renal tissue of patients with lupus nephritis, where it also correlates with disease activity (see “TNF and Organ Inflammation,” p. 18).6,7 In several lupus mouse models, including the MRL/lpr mouse, in particular, TNF significantly increases over time, in parallel with developing organ inflammation. Even NZB/W mice, which otherwise lack TNF, have increased TNF in their severely inflamed kidneys in the end. In some mouse model systems, attempts at TNF blockade have actually led to improvement in murine lupus.
Finally, the limited clinical experience with TNF blockade in SLE suggests benefits for inflammatory organ disease, including lupus nephritis, lupus arthritis, skin lupus, interstitial lung disease, and haemophagocytic syndrome.1,8 Of the first 10 patients treated in Vienna, all of whom were primarily treated with an induction regimen of four infusions of infliximab, eight had lupus nephritis and three had lupus arthritis (one had both). Two additional patients from Belgium and the U.K., with lupus nephritis and arthritis, and interstitial lung disease, respectively, were treated with somewhat different regimens.1 In addition, there are published case reports and small case series including 14 patients with lupus nephritis, 20 with lupus arthritis, and three with haemophagocytic syndromes.9-11
TNF Blockade and Findings of Lupus
Depending on the method of detection, a small but significant proportion of patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, or Crohn’s disease develop antibodies to dsDNA.2,19 Most of these antibodies are of the immunoglobin M isotype. Of the 2% of patients who develop high-affinity immunoglobin G anti-dsDNA antibodies, the majority develop lupus-like disease. Anti-TNF induced lupus may occasionally be severe, including organ manifestations such as nephritis, but is transient once TNF blockade is stopped.20
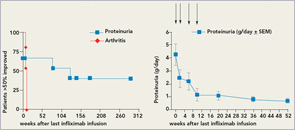
There appear to be significant differences in the response patterns of different organ manifestations in that effects were short-lived in lupus arthritis, comparable to the situation in RA, but long lasting (over years) in lupus nephritis and possibly in manifestations in other organs. Regarding treatment options, if these findings are confirmed, they would suggest the need for continuous therapy for lupus arthritis, but, much more favorably, only induction therapy for lupus nephritis (see Figure 1, below).
What has our limited clinical experience taught us about the safety profile of anti-TNF therapy in SLE? Unfortunately, this is not a simple question. Immunologically, an increase in lupus-specific autoantibodies has almost always been observed. However, we are not aware of a single lupus flare in association with such an increase at the time of anti-TNF therapy or shortly thereafter. Why flares do not occur is still unclear. The answer that we have observed too few patients probably will not hold muster anymore. The argument that anti-DNA antibodies developing during TNF may not be pathogenic appears implausible given that the antibodies are high affinity (Farr assay)
(CLIFT) antibodies to double-stranded DNA and occurred in patients who obviously made pathogenic antibodies before the TNF blockade.
One straightforward hypothesis to explain the presence of anti-DNA without nephritis is that TNF blockade protects against the negative effects of the antibodies. Another more elaborate theory invokes rapid changes in the levels or composition of immune complexes given their kinetics during and after TNF blockade. At the moment, we do not know why anti-DNA antibodies can occur without nephritis. Such a situation is not without precedent in lupus, however, and occurs with serologically active, clinically inactive lupus (i.e., high anti-DNA but no nephritis). The one immunologically mediated event seen and reported during anti-TNF therapy was deep venous thrombosis in a patient not on anticoagulation despite antiphospholipid syndrome; this event was associated with an increase in preformed (and presumably pathogenic) antiphospholipid antibodies. Thrombotic events thus may prove to be an important safety signal when TNF blockers are used for the treatment of lupus.
There are other unresolved issues. Infections are common and dangerous in patients with severe SLE, and experience suggests that this will also hold true under TNF blockade. Given the relatively low numbers of treated patients and potential reporting bias, numbers will not be significant to judge the impact on TNF blockade on susceptibility to infection in lupus. However, urinary tract infections during trials were quite common and might have been linked to proteinuric loss of anti-TNF antibodies. In patients under longer term TNF blockade, more serious and even one fatal infection (Legionella pneumonia) have been observed. In addition, one patient who was also on long-term anti-TNF treatment developed a cerebral lymphoma.10 Finally, after retreatment and in patients not concomitantly treated with azathioprine, mycophenolate, or methotrexate, severe infusion reactions occurred.1,12 Most of these safety issues could probably be minimized if anti-TNF therapy were restricted to induction therapy, as long-term TNF blockade in SLE appears to be less safe. Thus, a short course of induction treatment for lupus nephritis might have a more favorable risk–benefit profile than chronic treatment of lupus arthritis.
After observing the beneficial effects of TNF blockers in sick lupus nephritis patients, we believe that it would be a terrible shame if we forever lost the opportunity to study TNF blockade in a controlled clinical trial setting.
TNF Blockade and Lupus Nephritis
In lupus nephritis, recent clinical trial experience indicates the difficulty of demonstrating superiority of new therapies when compared with standard therapies. However, it is clear that there are patients for whom standard therapies are not effective. We believe that TNF blockade should primarily be tested in refractory lupus nephritis patients with high-grade proteinuria, given the available data. An induction regimen in combination with azathioprine or mycophenolate would be a reasonable option. From the infliximab experience, success was mostly achieved with high doses (5mg/kg), and occasional 3mg/kg therapy appeared insufficient. Thus, high-dose short-term therapy would be the goal. Anti-TNF therapy could either be added to mycophenolate induction in new onset lupus nephritis or used as a rescue approach in refractory lupus nephritis.
Although the combination of rituximab and MMF was not beneficial in a controlled clinical trial, the use of anti-TNF therapy in combination with MMF for new onset nephritis might be beneficial, given the much different kinetics of anti-TNF therapy and MMF, as long as time to response would count in assessing efficacy.13 However, as with the situation with essentially all biological response modifiers, the available experience is based on patients with nephritis refractory to standard therapy. Therefore, we advocate a randomized controlled trial of TNF blockade in class III, IV, or V lupus nephritis refractory to standard therapy.
TNF and Organ Inflammation
In MRL/lpr mice, TNF highly increases with time in the organs affected by lupus inflammation, in association with disease activity.21 Even in NZB/W mice, TNF is upregulated in late organ disease.22 TNF blockade with various agents improved organ inflammation in various mouse models, including arthritis and pneumonitis in MRL/lpr mice; arthritis, pneumonitis, and skin disease in motheaten mice; and nephritis in antibody-induced lupus in C3H.SW mice.23-25
The Role of Trials
Getting a prospective trial funded and successfully completed in the future will be a significant challenge. First, TNF blockers have gained such widespread popularity that it will likely be very difficult to convince any of the manufacturers to take a chance by studying these agents in a disease as risky and potentially problematic as SLE. Second, current standard therapy often includes cyclophosphamide, and there is strong reluctance to allow patients who have ever received cyclophosphamide into a TNF blocker trial. This reluctance is mostly based on the negative experience of Wegener’s Granulomatosis Etanercept Trial, in which an increased number of solid malignancies was found in those patients who received etanercept in combination with cyclophosphamide.14 However, we do not believe that concomitant therapy with both drugs is the same as sequential therapy.
Cyclophosphamide is an alkylating agent that produces DNA strand breaks. If malignant cells avoid death because TNF blockade takes away inflammation, tumors might result. This might even resemble the situation with ultraviolet light and TNF blockade in skin cancer, the only consistent pattern in TNF-induced cancer. In contrast, if a patient were to receive TNF blockade weeks or months after treatment with cyclophosphamide, malignant cells should have theoretically been removed, or they would have survived anyway. Only by resolving this important issue will a pivotal TNF blocker trial in lupus nephritis refractory to standard therapy have the chance to adequately recruit patients. Failure to recruit appropriate patients has already stopped the two trials in which we have participated: the European-based new-onset class V nephritis trial and the U.S.-based trial in proliferative nephritis, which excluded any patients who had ever used cyclophosphamide. Nevertheless, we still believe that an appropriate trial can and should be done.
After observing the beneficial effects of TNF blockers in sick lupus nephritis patients, we believe that it would be a terrible shame if we forever lost the opportunity to study TNF blockade in a controlled clinical trial setting. It is our hope that the lupus community will make a commitment to properly address this issue in the years to come and determine whether we are being are too reckless or too cautious in trying a therapy whose benefits in other diseases can be so impressive.
Dr. Aringer is a professor of medicine and chief of the division of rheumatology in the department of medicine III at the University Medical Center Carl Gustav Carus at the Technical University of Dresden, Germany. Much of the experience with TNF blockade in SLE stems from his earlier position as an associate professor in the department of rheumatology at the Medical University of Vienna in Austria. Dr. Dall’Era is associate professor of medicine and director of the Clinical Trials Center at the University of California, San Francisco.
Send Us a Letter!
CONTACT US AT:
David Pisetsky, MD, PhD, physician editor
E-mail: [email protected]
Dawn Antoline, editor,
E-mail: [email protected]
Phone: (201) 748-7757
The Rheumatologist welcomes letters to the editor. Letters should be 500 words or less, and may be edited for length and style. Include your name, title, and organization, as well as a daytime phone number.
References
- Aringer M, Houssiau F, Gordon C, et al. Adverse events and efficacy of TNF-alpha blockade with infliximab in patients with systemic lupus erythematosus: Long-term follow-up of 13 patients. Rheumatology (Oxford). 2009;48:1451-1454.
- Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: Findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 2000;43:2383-2390.
- Aringer M, Steiner G, Graninger WB, Hofler E, Steiner CW, Smolen JS. Effects of short-term infliximab therapy on autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 2007;56:274-279.
- Studnicka-Benke A, Steiner G, Petera P, Smolen JS. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35:1067-1074.
- Aringer M, Feierl E, Steiner G, et al. Increased bioactive TNF in human systemic lupus erythematosus: Associations with cell death. Lupus. 2002;11:102-108.
- Aringer M, Smolen JS. Cytokine expression in lupus kidneys. Lupus. 2005;14:13-18.
- Zampieri S, Alaibac M, Iaccarino L, et al. Tumour necrosis factor alpha is expressed in refractory skin lesions from patients with subacute cutaneous lupus erythematosus. Ann Rheum Dis. 2006;65:545-548.
- Ideguchi H, Ohno S, Takase K, et al. Successful treatment of refractory lupus-associated haemophagocytic lymphohistiocytosis with infliximab. Rheumatology (Oxford). 2007;46:1621-1622.
- Aringer M, Smolen JS. Therapeutic blockade of TNF in patients with SLE—promising or crazy? Autoimmun Rev. 2010. In press.
- Aringer M, Graninger WB, Steiner G, Smolen JS. Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: An open-label study. Arthritis Rheum. 2004;50:3161-3169.
- Uppal S, Hayat S, Raghupathy R. Efficacy and safety of infliximab in active SLE: A pilot study. Lupus. 2009;18:690-697.
- Katz RS, Holt-Daly N, MacDonald PA. Frequent infusion reactions associated with infliximab treatment in patients with polyarthritis related to systemic lupus erythematosus. Arthritis Rheum. 2003;48(Supplement):S379.
- Furie R, Looney RJ, Rovin B, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): Results from the randomized, double-blind phase III LUNAR trial. Arthritis Rheum. 2009; 60(Supplement):S429.
- Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352 :351-361.
- Jacob CO, McDevitt HO. Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature. 1988;331:356-358.
- Gordon C, Ranges GE, Greenspan JS, Wofsy D. Chronic therapy with recombinant tumor necrosis factor-alpha in autoimmune NZB/NZW F1 mice. Clin Immunol Immunopathol. 1989;52:421-434.
- Kontoyiannis D, Kollias G. Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur J Immunol. 2000;30:2038-2047.
- Jacob N, Yang H, Pricop L, et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol. 2009;182: 2532-2541.
- Aringer M, Smolen JS. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. 2008;10:202.
- De Bandt M, Sibilia J, Le L, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: A French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050-3054.
- Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989;143:3470-3475.
- Su X, Zhou T, Yang P, Edwards CK, Mountz JD. Reduction of arthritis and pneumonitis in motheaten mice by soluble tumor necrosis factor receptor. Arthritis Rheum. 1998; 41(1):139-149.
- Edwards CK, III, Zhou T, Zhang J, et al. Inhibition of superantigen-induced proinflammatory cytokine production and inflammatory arthritis in MRL-lpr/lpr mice by a transcriptional inhibitor of TNF-alpha. J Immunol. 1996;157:1758-1772.
- Segal R, Dayan M, Zinger H, Mozes E. Suppression of experimental systemic lupus erythematosus (SLE) in mice via TNF inhibition by an anti-TNFalpha monoclonal antibody and by pentoxiphylline. Lupus. 2001;10:23-31.
