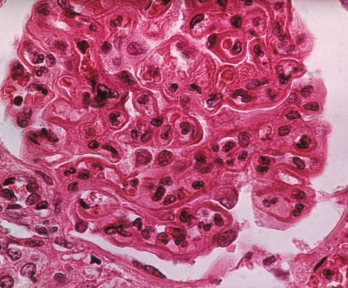
The pattern of lupus glomerular involvement in lupus nephropathy is similar to that in membranous nephropathy, forming wire-loops lesions.
Biophoto Associates / Science Source
Researchers who devote their time to studying lupus are accustomed to considering environmental stimuli such as sunshine and cigarettes. But according to Gregg J. Silverman, MD, a professor in the Department of Medicine and in the Department of Pathology at the New York University (NYU) School of Medicine and co-director of the Musculoskeletal Center of Excellence, we should be taking a closer look at the internal environment: the gut microbiome.
Dr. Silverman and colleagues set out to do a thorough search for a transmissible agent in systemic lupus erythematosus (SLE). Their work, “Lupus Nephritis Is Linked to Disease-Activity Associated Expansions and Immunity to a Gut Commensal,” appears in the Feb. 19, 2019, online edition of the Annals of Rheumatic Diseases.1
Consider the Internal Environment
“Lupus is one of the most fascinating and challenging diseases you can imagine,” says Dr. Silverman, a practicing rheumatologist who is also a molecular immunologist. “On one level, it is an autoimmune condition where the body attacks itself—but it does so in specific patterns. The hallmark of lupus is that these patients make anti-nuclear antibodies (ANA). When there are anti-double-stranded DNA antibodies present, it often signals the development of the most serious complications of lupus. These patients are experiencing an immunological reaction to the building blocks of life.”
Dr. Silverman says research findings related to identical twins are puzzling. If one twin develops lupus, the other twin develops the disease only 25–30% of the time. “Roughly 20 years ago I had a patient who, after about two years of treatment for her lupus, told me that she had a twin. Both siblings donated blood and thus began my initial research on twins and the immune system. Whenever I see a lupus patient I now ask, ‘Perhaps it’s not always fated to happen. What’s going on in this person’s internal environment that might contribute?’”
Dr. Silverman began his translational science work at NYU in 2011. “The hunt for a transmissible agent in lupus became feasible due to the explosion of technology related to the human genome initiative,” he says. “Not only did it become less expensive to determine DNA sequences, but the software became available to sift through all of the microbiome DNA data.”
A Big Population That Bears Investigation
Dr. Silverman, notes, “The discovery that every one of us has three to 10 times more microbial cells than human cells in our body lit the fuse for me, as well as for other investigators. The gut microbiome is a vast, undiscovered world that holds promise for treating numerous diseases. For our purposes, we matched blood and fecal samples from 61 female patients with SLE, using 17 female healthy controls. We undertook fecal 16S rRNA analyses that examined genes from each microbe present in the bowel, and we performed sera profiling for antibacterial and autoantibody responses.”
Dr. Silverman says they learned that lupus patients have reduced gut microbiome diversity than healthy controls and varying levels of dysbiosis. “However, few efforts have been made to characterize the microbiome of those with active lupus,” he says.
The Interesting Glitch
“Physicians find lupus so difficult to a great extent because the disease manifests differently in different individuals,” Dr. Silverman says. “Add in the variability of disease activity—remission, exacerbations and progression—and you have a perfect storm of clinical challenges.
“I reflect deeply on each case, asking myself, ‘Who are these patients, and how are they affected by the disease?’ We have data with which we can do a sophisticated computational analysis of the microbiome … and when doing so I always consider that set of twins from many years ago. What would they look like? Would the microbiome in a healthy (non-active disease) patient be different than one in someone with active lupus? Some of the patients in our study were very ill, while others were feeling well [i.e., were not taking medication and had no detectable disease activity].
“I knew that what we were undertaking was not going to be a simple analysis where we would compare controls to people with disease,” Dr. Silverman explains. “I decided that we needed three groups: healthy patients, those with active lupus and those with inactive lupus.”
‘We found the microbiome of patients with SLE also showed decreased species richness diversity, with the most significant reductions in taxonomic complexity in patients with high disease activity.’ —Dr. Silverman
Pattern Identification
Those were the first threads of an effort that resulted in the identification of a pattern in which the presence of a single type of bacteria was expanded in the intestines of lupus patients. The more of the bacteria that was present, the sicker the patients were.
“We found the microbiome of patients with SLE also showed decreased species richness diversity, with the most significant reductions in taxonomic complexity in patients with high disease activity [as measured by the SLE disease activity index; SLEDAI],” Dr. Silverman says.
“Particularly interesting was the finding that patients with SLE had a fivefold greater representation of Ruminococcus gnavus [RG] in their guts. While RG is not necessarily bad or uncommon, we found that if one particular strain exists in a lupus patient then that person will become sick,” he explains.
“We found that some strains produce a molecule called a lipoglycan. While in some individuals the lipoglycan could normally be innocuous and sealed in the intestinal tract, we believe that in these cases, it is thrown off in the local intestines and released because of a leaky gut. We found that not only do lupus patients have more of this bacteria [RG], but that the patients exhibit a strong antibody response to this type of molecule in one species. So in patients who develop kidney disease, some part of the bowel is like a screen door … things are getting let out, which may be driving the autoantibody response that leads to nephritis.
“At this point,” Dr. Silverman says, “we have been able to develop enough data along with a testable hypothesis that perhaps lupus patients have too much of this one bad strain of bacteria. Going forward, we should be developing early testing for this bacterium, its lipoglycan and antibodies to the lipoglycan. The next goal would be to eliminate the released lipoglycan.”
Commenting on the research process, Dr. Silverman notes, “At certain points I was a bit discouraged because all of the patients were so different from one another. Some were experiencing joint issues while others had rashes—and the rashes often differed from patient to patient. I thought, ‘Is lupus really one disease?’
“It looked like so many different conditions that I was reminded of the … story where seven blind men each touched different parts of an elephant, and each thought it was something different,” he says. “It was tenacity that kept us going. Using the SLEDAI scale was a bit of a risk, because it is rather simple and not meant to be used for the microbiome. We borrowed tools during this exploratory process and luckily, they turned out to be relatively useful. That both active and inactive lupus patients could be involved in one study and get such clarity was unexpected and very promising.”
The Lupus-Gut Connection
Dr. Silverman says his team is cloning bacteria from the intestines of lupus patients. “It is amazing to be able to do whole-gene sequencing on these bacteria, learn their secrets and determine how they contribute to illness,” he says. “We are also looking at patients at multiple time points, asking, ‘Do patients remain stable with the same microbiome over time? How is this related to the immune system?’ Our goal is to be able to obtain a more accurate diagnosis—and to do so in a timely fashion.”
The next steps are to confirm the findings from this study in other lupus populations. “Ideally, this kind of testing can tell us whether someone is likely to progress to kidney disease,” he says. “It is important that we look at how to shift the microbiome away from bad bacteria; this may involve fecal transplants. While, thus far, this treatment has been successful in many patients with a bowel disease … we are not ready to attempt it in patients with SLE.”
The takeaway for practicing rheumatologists? “This work can help bring hope to patients that perhaps there are simple things we can do to improve their conditions,” Dr. Silverman says. “In the future, this may lead to therapies that would stand in great contrast to the current standard of care for lupus nephritis, which involves escalating medications that suppress the immune system—cancer therapy that basically kills the immune system.”
Elizabeth Hofheinz, MPH, MEd, is a freelance medical editor and writer based in the greater New Orleans area.
Reference
- Azzouz D, Omarbekova A, Heguy A, et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis. 2019 Jul;78(7):947–956.