The concept of difficult-to-control gout describes several clinical head-bangers.
It may refer to failure to achieve the recognized target serum urate level of <6.0 mg/dL despite adequate therapeutic efforts with urate-lowering agents. It can refer to failure to suppress or stop gout flares, or it can describe failure to prevent the disease progression. All too often, all three collide.
The Stumbling Blocks
The causes of difficult-to-control gout fall on the shoulders of both patients and the medical professionals caring for them.
On the patient side, previous negative experiences with gout therapies; nonadherence to complex treatment plans; and unwillingness to curtail behavioral patterns, such as daily beer drinking, often compound a poor understanding of which medication is used for which feature of gout.
Many gout flares are seen in emergency departments or primary care practices where clinicians are often unaware of best practices in gout management when comorbidities feature strongly. They frequently fail to make the diagnosis, may trivialize intermittent flares and are often late to initiate urate-lowering therapy. Often, they lack the time to embrace a high-intensity approach, which is usually needed to control the hyperuricemia and gout. Treating to a serum-urate target is not yet well integrated into primary care practice. The concept of providing antiinflammatory coverage to mitigate flares following the initiation of urate-lowering therapy is still new to many.
There is a widespread failure of both patients and clinicians to understand contemporary gout treatments. Newly published guidelines do articulate the distinct goals of urate-lowering therapy, gout flare prophylaxis and acute flare pain control, but a big disconnect remains between the guidelines and clinical practice.
Many patients with hyperuricemia and gout are perceived as being too complex to treat effectively. However, it’s just those patents who should be referred for rheumatologic care.
This case highlights many of the challenges facing patients and clinicians.
The Case
EH was a 60-year-old white man with a 10-year history of gout, which first manifested as episodic, acute-onset flares of monoarthritis in the lower extremities with pain and swelling. These typically lasted four to seven days. He would try to self-medicate using acetaminophen or low doses of nonsteroidal antiinflammatory drugs (NSAIDs). When this approach failed to alleviate his symptoms, he would go to an emergency department. He never discussed these events with his primary care physician, whom he saw infrequently.
Five years ago, he was referred to our rheumatology clinic by our emergency department after an especially long flare, his third gouty attack that year.
When we saw him, he had no other medical problems, and his blood pressure and BMI were normal. His peripheral joints were all cool with full range of motion. He had no obvious tophi. His serum acid was 10.2 mg/dL. His renal function studies were normal. Risk factors included a family history of gout (i.e., his father) and heavy beer consumption, especially on weekends. He was a long distance truck driver.
We used this visit to educate him about gout, describing it as a chronic condition of excess urate burden, and emphasized our approach to management, stressing both short- and long-term goals. We hoped this would reduce future costly visits to the emergency department.
He had already started taking colchicine 0.6 mg twice daily. We initiated allopurinol 100 mg daily with the goal of reducing his serum uric acid to less than 6.0 mg/dL. With slow allopurinol titration and continued flare prophylaxis, his uric acid slowly fell to 8.0 mg/dL.
Unfortunately, EH stopped coming to the clinic. Not surprisingly, his allopurinol prescription ran out, and his uric acid was never checked again. This is not an unusual situation for patients suffering from episodic attacks of gout. They wait until the pain returns before seeking medical attention.
Five years later, he enrolled with a primary care physician. By then he was experiencing monthly gouty flares affecting the great toes, the mid foot, the knees and the elbows. His job as a long-haul truck driver was becoming compromised.
Follow-up
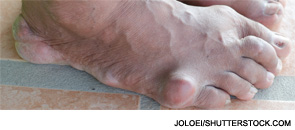
EH was referred back to our rheumatology clinic by his primary care physician. He admitted that he had not taken allopurinol or colchicine for years. Instead, he had continued to seek flare-based episodic care in various parts of the country. This often consisted of intramuscular corticosteroids or methylprednisone dosepacks. At our visit, he had a significant knee effusion. Aspiration and synovial fluid analysis confirmed the presence of monosodium urate crystals. His uric acid that day was 10.2 mg/dL. Erosive changes in his right great toe were seen on X-ray. Showing him these images proved quite useful.
Once again, colchicine 0.6 mg BID and allopurinol 100 mg daily were restarted. A tapering dose of prednisone was added to provide immediate relief. He was counseled to curtail his beer drinking, and he freely admitted that this would be his toughest task.
Three weeks later he returned, feeling better, but with a resolving right elbow effusion. His uric acid was 11.0 mg/dL He was taking only 5 mg of prednisone daily. He had stopped the allopurinol because of nausea and the colchicine because he was afraid it would cause diarrhea. He had experienced significant diarrhea during one of his prior emergency department visits when he was prescribed an excessive dose of colchicine.
Once again, it became important for our team to come up with a plan that he would adhere to and that would provide relief. We initiated febuxostat 40 mg daily. We convinced him to try taking just two colchicine tablets daily. We added vitamin C to his daily regimen to exploit its mild uricosuric effect. We kept him on a 5 mg dose of prednisone. Perhaps most importantly, we decided that his flares were so hard to control that we started him on anakinra injections three times a week.
He called our office three weeks later to tell us that he was thrilled to be flare free. We advised him to taper off the prednisone, but to keep taking the colchicine and febuxostat. We tapered the anakinra to a weekly injection.
Several weeks later, he remained symptom free, and his uric acid had dropped to 6.8 mg/dL. We increased his febuxostat to 80 mg daily.
We saw him three months later. His weight and blood pressure were stable. He was not drinking alcohol. He was taking febuxostat 80 mg daily and colchicine 0.6 mg BID. He was off corticosteroids and anakinra. The serum uric acid was 5.2 mg/dL. He tolerated this regimen well.
Our plan was to continue seeing him at four-month intervals to monitor therapy, and we eventually weaned him off colchicine.
We continued to spend time stressing the key educational aspects about gout. These are the points that most primary care doctors are often unable to review because of a lack of time or unfamiliarity with current guidelines for the care of hyperurecemia and gout. We emphasized that we were always available to help him manage an acute flare, but that he really needed to stay compliant with his current regimen and his current diet if he wanted to reduce the risk of future gouty flares and other complications of gout and hyperurecemia.
Conclusion
This case is probably not unusual for rheumatologists to see. Gout management is often crisis driven. There is episodic use of the healthcare system with limited or infrequent follow-up care. Often, patients’ compliance with care is limited by many factors. Rheumatologists can provide intensive, evidence-based care so that even refractory, difficult-to-treat cases of gout become manageable. It just takes time and patience.
Joan McTigue is a physician assistant for the rheumatology section of the VA Medical Center in Gainesville, Fla. She staffs the Gout Clinic there with rheumatology faculty and fellows of the University of Florida College of Medicine. In her spare time, she participates in expedition mountaineering.


