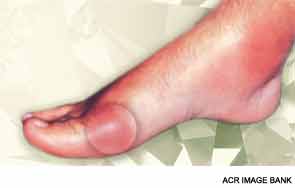
The incidence of gout has been steadily increasing during the past few decades, from about 0.5% of the population in the early 1970s to about 4% as of 2010, making it increasingly important to provide quality care to this patient population. To improve the quality of gout care, both physicians and patients need to understand the goals and effects of gout therapy, said Michael H. Pillinger, MD, associate professor of medicine and biochemistry and molecular pharmacology, NYU School of Medicine, New York, at the ACR’s Winter Rheumatology Symposium this January in Snowmass, Colo. During the session, Update on Gout Therapy: The ACR 2012 Treatment Guidelines and Beyond, Dr. Pillinger discussed current treatment options and the recently published ACR guidelines.
Quality of Care
Overall, the quality of gout care today is poor, Dr. Pillinger said. “A very big underlying reason [for this] is a lack of understanding on the [part] of both patients and physicians about the nature of the disease. Managing attacks is the tip of the iceberg,” he explained. Although the pain from a gout attack can be treated with nonsteroidal antiinflammatory drugs (NSAIDs), failing to manage gout effectively tends to make the gout worse over time and increases the frequency of attacks.
Another complication is that the consequence of treating gout properly is that it will actually make the patient worse for a period of time, Dr. Pillinger said. If the patient is not treated with antiinflammatory medication in addition to urate-lowering therapy during the “vulnerable window,” which can last 6–24 months or longer, the patient is likely to blame the gout medication for increased attacks and stop taking it.
It’s also important to “treat to target,” Dr. Pillinger said. It’s not enough to put the patient on urate-lowering therapy; follow-up is needed to ensure the patient is progressing toward an appropriate urate level. The keys to quality treatment are “understanding and knowing the nature of the targets, and recognizing that gout is a long-term disease not a short-term disease,” Dr. Pillinger said. “One of the main goals of the ACR treatment guidelines is to lay this out for practitioners, and they’re valuable in that regard.”
ACR Gout Treatment Guidelines
The 2012 American College of Rheumatology Guidelines for Management of Gout were published after a process of literature review and appraisal. During the review process, core expert panels (CEPs) and task force panels (TFPs) convened to investigate four areas of interest: therapy of acute gouty arthritis, urate lowering, antiinflammatory prophylaxis and management of chronic tophaceous gouty arthropathy. To determine whether each recommendation would be included in the guidelines, the panel members assigned ratings independently, then shared the ratings, discussed and voted. Recommendations that are included in the guidelines also include quality ratings to indicate the level of evidence that supports the practice.
Rheumatologists are encouraged to consider the evidence, account for the individuality of their patients and make their own decisions for providing care, Dr. Pillinger said. “The experts may wish to bend the rules,” he added, “but the guidelines needed to be clear enough that nonexperts could use them for guidance in the right circumstances.”
Acute Attacks
The primary recommendations for managing an acute gouty attack, Dr. Pillinger said, are pharmacologic therapy that is initiated within 24 hours of acute attack onset and not interrupting ongoing pharmacologic urate-lowering therapy during an acute attack.
The first line of treatment is using monotherapy or combination therapy. Monotherapies include NSAIDs, COX-2 inhibitors, corticosteroids or colchicine. In the event of inadequate response to monotherapy, an alternative monotherapy or add-on combination therapy can be considered.
In the event that monotherapy and combination therapy are ineffective, off-label therapy with anti-interleukin (IL) 1 treatment may be considered. “Over the last five or six years, it’s come to be understood that a fundamental thing uric acid crystals do when they activate the white cells is activate the inflammasome,” Dr. Pillinger explained. “And this puts IL-1 at the center of the inflammatory process of gout.”
There are three biologic agents available that interfere with IL-1: anakinra, rilonacept and canakinumab. The safety of these agents has been established in a few diseases, although gout is not one of them and they are not FDA approved yet, Dr. Pillinger said. He shared research results in support of these medications for gout:
- Anakinra has demonstrated benefit in gouty attack.
- Canakinumab is equally effective as the standard European therapy of a single injection of triamcinolone.
- Rilonacept has a dose-dependent effect on reducing total flares during urate-lowering therapy.
Established Gout
The baseline plan for managing established gout starts with the following initial considerations:
- Implement dietary and lifestyle alterations (e.g., weight loss for obese patients, healthy overall diet, exercise/activity, smoking cessation, staying well hydrated).
- Consider secondary causes of hyperuricemia (comorbidity checklist).
- Consider eliminating nonessential medications that promote hyperuricemia (e.g., niacin, thiazide and loop diuretics, cyclosporine, tacrolimus; not aspirin). However, particularly in the case of diuretics, the committee emphasized that these drugs may be important in the individual patient and should not be universally discontinued.
- Clinically evaluate gout disease burden/severity (i.e., palpable tophi, frequency and severity of attacks, chronic symptoms and signs).
Dietary considerations are important for patients with established gout. Among the recommendations are to avoid high-purine organ meats and high-fructose corn syrup, limit serving sizes of naturally sweet fruit juices and table sugar, and encourage low- or non-fat dairy intake.
Dairy is of interest because it shows promise for reducing urate levels. “The more dairy you eat, the less gout you have,” Dr. Pillinger said. “And that holds after you adjust for meat consumption. It looks like something or other in dairy products drives the kidneys to excrete more uric acid.”
The baseline plan for managing established gout also includes indications for urate lowering. One key with urate-lowering therapy is to “treat to target,” Dr. Pillinger said. “The minimum serum urate target is less than 6.0 mg/dL, and serum urate lowering below 5 mg/dL may be needed to improve gout signs and symptoms.”
Use of an XOI, either allopurinol or febuxostat, is the recommended first-line therapy for urate lowering. An alternative first-line therapy, if at least one XOI is contraindicated or not tolerated, is the uricosuric probenecid. Probenecid is not considered a first-line therapy because it isn’t as effective as the XOIs, and there are limitations for using it, Dr. Pillinger noted.
“[Probenecid] doesn’t work well in people with any more than mild kidney disease because it relies on renal function to work,” Dr. Pillinger said. “Additionally, probenecid can raise the risk of kidney stones, particularly in patients with a history of them. Probenecid gets rid of urate by pushing it into the renal pelvis and into the urine,” he said. “You concentrate it in the kidney where it can precipitate. You have to drink a lot of water and may have to take medicine to keep the urine alkalotic.”
Prophylaxis
It’s extremely important to initiate prophylaxis along with urate-lowering therapy, Dr. Pillinger said. The ACR recommends low-dose colchicine (0.5–0.6 qD or BID) or low-dose NSAIDs (e.g., naproxen 250 BID) for first-line therapies and prednisone equal to or less than 10 mg/day as a second-line therapy.
Another important recommendation is to periodically assess for gout activity (e.g., acute attacks, persistent tophi, persistent chronic synovitis) during treatment. Prophylaxis should be continued if any disease activity is still present.
“Don’t stop the prophylaxis until the risk of attacks has gone away, and that’s characterized by getting rid not only of hyperuricemia, but also of the attacks themselves, as well as visible tophi and occult urate deposits, and then giving time to allow the dust to settle,” Dr. Pillinger said. “This will be four to five months minimum, and the maximum could be several years. This a very important recommendation.”
Kimberly J. Retzlaff is a medical journalist based in Denver.

