Following treatment with pegloticase, dual-energy CT images were obtained, and the images were compared with those done the prior year. Beyond the multiple erosions, the images showed extensive tophi and deposition in the flexor and extensor tendons of hands, ankles and feet (see Figure 2 and Figures 3–5). Based on the persistently elevated PUA levels (with no value ever recorded less than 6 mg/dL) and the unchanged tophi, our patient was deemed to have failed pegloticase, and the treatment was stopped after approximately five months.
Discussion
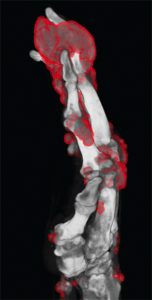
Figure 3: DECT of hands #2
Pegloticase is a mammalian PEGylated recombinant form of uricase that catalyzes the oxidation of uric acid to allantoin and is used in cases of severe refractory gout. In prior studies, it has been shown to significantly lower PUA and reduce tophi burden by more than 80% in those who were able to maintain PUA less than 6 mg/dL.1-2 However, not all patients treated with pegloticase maintain their response, and some begin to lose their response, leading to increased PUA and infusion reactions. This can be due to a variety of reasons, including antibody formation, as well as genetic predisposition and abnormalities in the renal urate transport system.
It should be noted that in initial and subsequent studies, all patients given pegloticase initially had significant decreases in their PUA.2-4 In a study by Sundy et al, it was noted that nonresponders in the bimonthly treatment groups still had a mean PUA of less than 6 mg/dL until Week 10 and in the monthly treatment group until Week 5.2 In our particular case, the patient never once obtained a PUA of less than 6 at any point during his five-month treatment course (lowest recorded value was 8.1 mg/dL) or had any clinically detectable decrease in tophi burden.
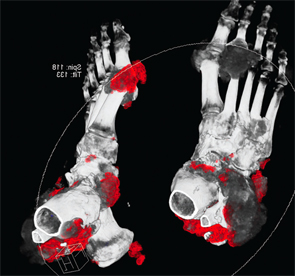
Figure 4: DECT of feet #1
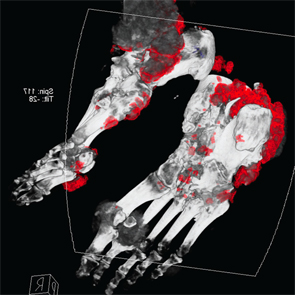
Figure 5: DECT of feet #2
One known reason for failure of pegloticase and decreased response over time is antibody production. Three known antibodies have been described in the literature: anti-PEG, anti-pegloticase and anti-uricase.
In a study by Lipsky et al, it was shown that these antibodies play a clinically significant role in pegloticase clearance and that 89% of patients had anti-pegloticase antibodies after the first infusion.4 However, in those who responded to pegloticase (defined by PUA level less than 6 mg/dL for 80% or more of the three- to six-month study period), they typically had low titers of the antibody (<1:2,430). Additionally, during the first two months of treatment, responders showed a tendency toward formation of IgM antibodies and not IgG, whereas nonresponders typically had IgG formation by Week 3, as well as high titers of anti-pegloticase antibodies.