The ACR recently released two new clinical practice guidelines pertaining to the management of juvenile idiopathic arthritis (JIA).1,2 Among their key recommendations are decreased reliance on long-term corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs), early use of biologic and conventional disease-modifying anti-rheumatic drugs (DMARDs) and an emphasis on shared decision making with patients and caregivers.
Part 2 of the JIA Update
The ACR released initial guidelines for JIA in 2011 and 2013, and began an update process in 2017 in response to the rapidly changing nature of the field, which included the approval of new treatments and increased clinical experience using others.3,4 Due to the breadth of topics that needed to be covered, including broad clinical manifestations seen in the seven different subtypes of JIA, the work was eventually split into four papers.
The two papers published in 2019 covered non-systemic polyarthritis, sacroiliitis, enthesitis and uveitis. The other two guidelines were recently published. One addresses oligoarthritis, temporomandibular joint (TMJ) arthritis and systemic JIA, with and without macrophage activation syndrome (see Figures 1–3).5 The other provides general recommendations with respect to all JIA types for nonpharmacological treatments, medication monitoring, immunizations and imaging.6
Relevant clinical questions were first developed using the PICO (i.e., population, intervention, comparator and outcomes) format. After a comprehensive literature review, a panel of adult patients and patient family members provided their perspectives to a voting panel, which included two of the patient panel members. Voting panel members then provided specific conditional or strong recommendations using GRADE (i.e., Grading of Recommendations, Assessment, Development and Evaluation) methodology, which has been used for other recent ACR guidelines.

Dr. Onel
Karen B. Onel, MD, chief of the Pediatric Rheumatology Division at the Hospital for Special Surgery, New York City and the lead investigator for the two most recent JIA guidelines, notes that the evidence base for pediatric rheumatology is extremely limited, with none of the recommendations supported by moderate to high-quality evidence. However, existing literature and literature from closely related diseases heavily inform these recommendations.
Key Themes
Escalate treatment when appropriate
Another voting panel member, Randy Q. Cron, MD, PhD, director of the Division of Pediatric Rheumatology, the University of Alabama at Birmingham, notes that part of the guideline focus was to recommend escalating to biologics more rapidly than many clinicians have done in the past to provide faster and better disease control with fewer side effects.

Dr. Cron
“Steroids are powerful immunosuppressive medications that help our children in a lot of ways,” he says, “but they have so many shortcomings and long-term side effects that anything you can do to get patients onto a lower dose and/or off them is a good thing. That often means earlier adoption of DMARDs and, especially, biologic DMARDs for patients with some of our diseases.”
Dr. Onel remarks on the incredible progress that has been made in treating these conditions in the past 30 years, when glucocorticoids were the treatment mainstay. “All those hip replacements, all of those kids who were unnaturally short, who had terrible bones—we don’t see that anymore.” But she thinks we still might do a better job of escalating treatment when necessary because non-pediatric rheumatologists, in particular, tend to undertreat and rely too heavily on steroids and NSAIDs.7
“We are trying to say in these guidelines that children tolerate medicines,” she says. “We provide guidance on how to monitor them and keep them safe, but you must treat them adequately.”
Emphasis on shared decision making
These guidelines also place a strong emphasis on the need for shared decision making among caregivers, patients and clinicians. Such an emphasis is perhaps even more important in a context such as JIA, which relies on a lower-quality-evidence base than some conditions and which contains many more conditional (vs. strong) recommendations. “We have options about which drug to choose and when to start them,” says Dr. Cron, “so having [patient and caregiver] input is valuable.”
Recommendations
Some of the key recommendations in the new JIA guidelines are described below. See the complete guideline documents for additional recommendations, details and context.1,2
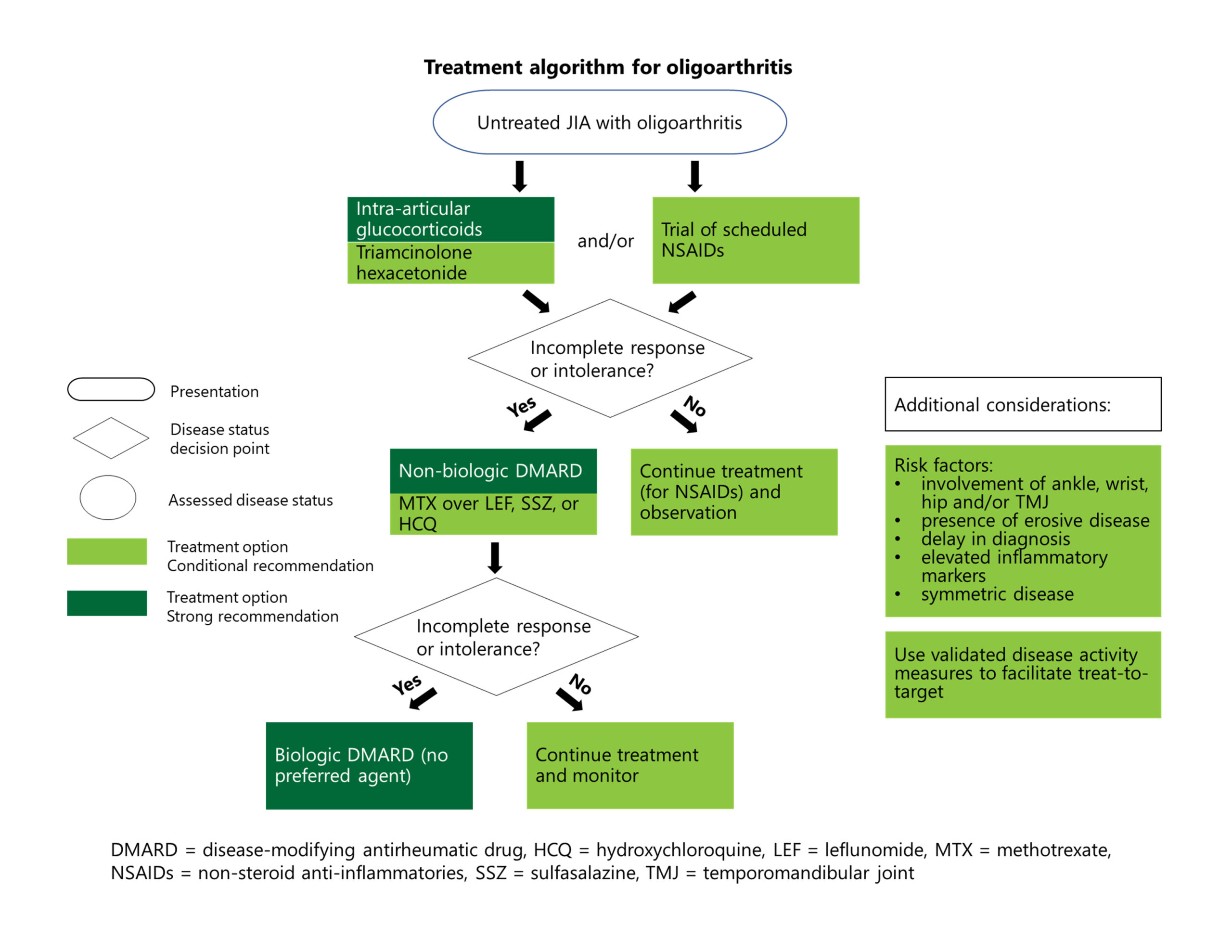
Treatment for Active Oligoarthritis
Recommendation: For initial therapy for patients with active disease, the panelists conditionally recommend a brief trial of NSAIDs as part of therapy. Note: The guideline writers chose not to include recommendations on specific time frames for treatment escalation for oligoarthritis or the other JIA subtypes discussed. “The point we were trying to make is that if [a therapy] isn’t working, you need to move on—especially if the patient is getting worse,” says Dr. Onel.
Recommendations: The guidelines strongly recommend against oral glucocorticoids, but strongly recommend in favor of intra-articular glucocorticoids.1
Intra-articular steroids are appealing for their low potential for causing adverse effects and high likelihood of a sustained response. Triamcinolone hexacetonide, a long-acting version, has been unavailable in the U.S. for many years, but the U.S. Food & Drug Administration recently allowed it specifically for joint injections in patients with JIA.
“Now that we have this formulation of triamcinolone hexacetonide available, it’s probably going to pick up use again,” explains Dr. Cron, “because the second-best intra-articular steroid may only last two months to keep the arthritis at bay, whereas this one may last a year.”
Intra-articular triamcinolone hexacetonide may be particularly helpful for patients who have only one or two joints with active disease at presentation, or if a patient has one or two joints that haven’t responded to other interventions, he notes.
Recommendations: After a brief trial with NSAIDs, if the disease is still inadequately treated or if treatments are not tolerated, conventional synthetic (cs) DMARDs are strongly recommended. If the disease is still not adequately controlled, biologic (b) DMARDs are strongly recommended.1
However, the guideline writers were careful to point out that this rough treatment algorithm should be interpreted in the clinical context and modified, if needed. For example, providers may need to rapidly escalate therapies in the context of poor diagnostic features, such as elevated inflammatory markers, erosive disease, enthesitis or involvement of the ankle, wrist, hip, sacroiliac or temporomandibular joints.
Although methotrexate is recommended over other csDMARDs, the guideline writers expressed no preference in terms of bDMARDs, given the lack of available comparative evidence.
Dr. Onel notes that tumor necrosis factor (TNF) inhibitors are the most commonly used bDMARDs in childhood arthritis, so we have the most clinical experience and data about their safety and effectiveness. However, given the limited number of head-to-head trials of biologics, other bDMARDs, such as abatacept or interleukin (IL) 6 inhibitors, could be used in this context.
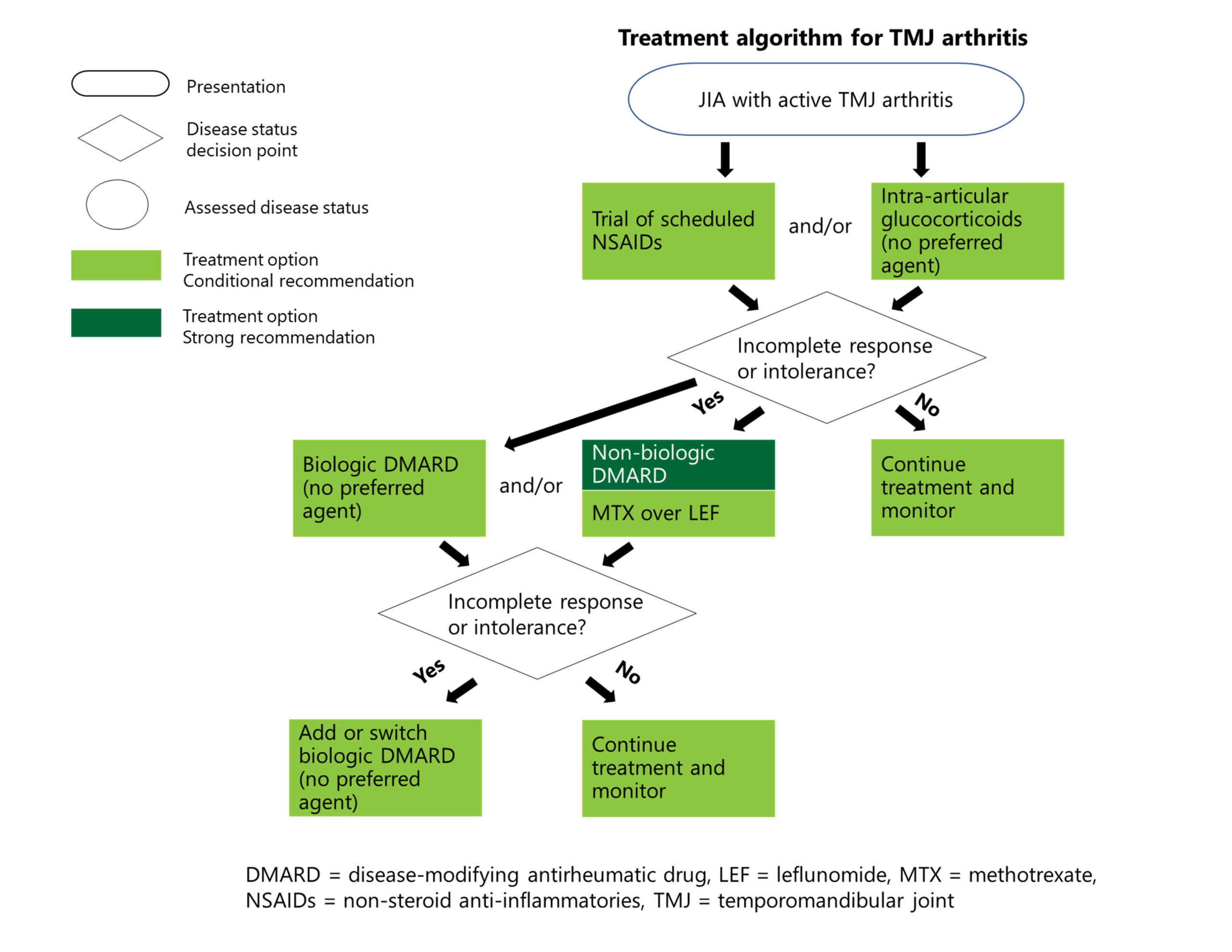
Treatment for TMJ Arthritis
Recommendations: For patients with active disease, the panelists conditionally recommend a brief trial of NSAIDs as part of the initial therapy, conditionally recommend against oral glucocorticoids, but conditionally recommend in favor of intra-articular glucocorticoids.1
Recommendations: If symptoms are still not well controlled after treatment with NSAIDs and/or intra-articular glucocorticoids, csDMARDs are strongly recommended. bDMARDs are conditionally recommended at this point or after inadequate response to csDMARDs.1
TMJ arthritis can occur alone or in tandem with arthritis in other joints. Dr. Onel points out that the guideline writers wanted to include specific recommendations on TMJ because of its potentially devastating complications. “You just can’t overstate how important it is that people be able to open their mouths. It definitely needs to be treated aggressively.”
TMJ arthritis has powerful deleterious growth effects on children, on the midface as well as the jaw, Dr. Cron adds. “It is a very commonly involved joint, but one that is very difficult for us to treat; it remains one of our problematic joints,” he says.
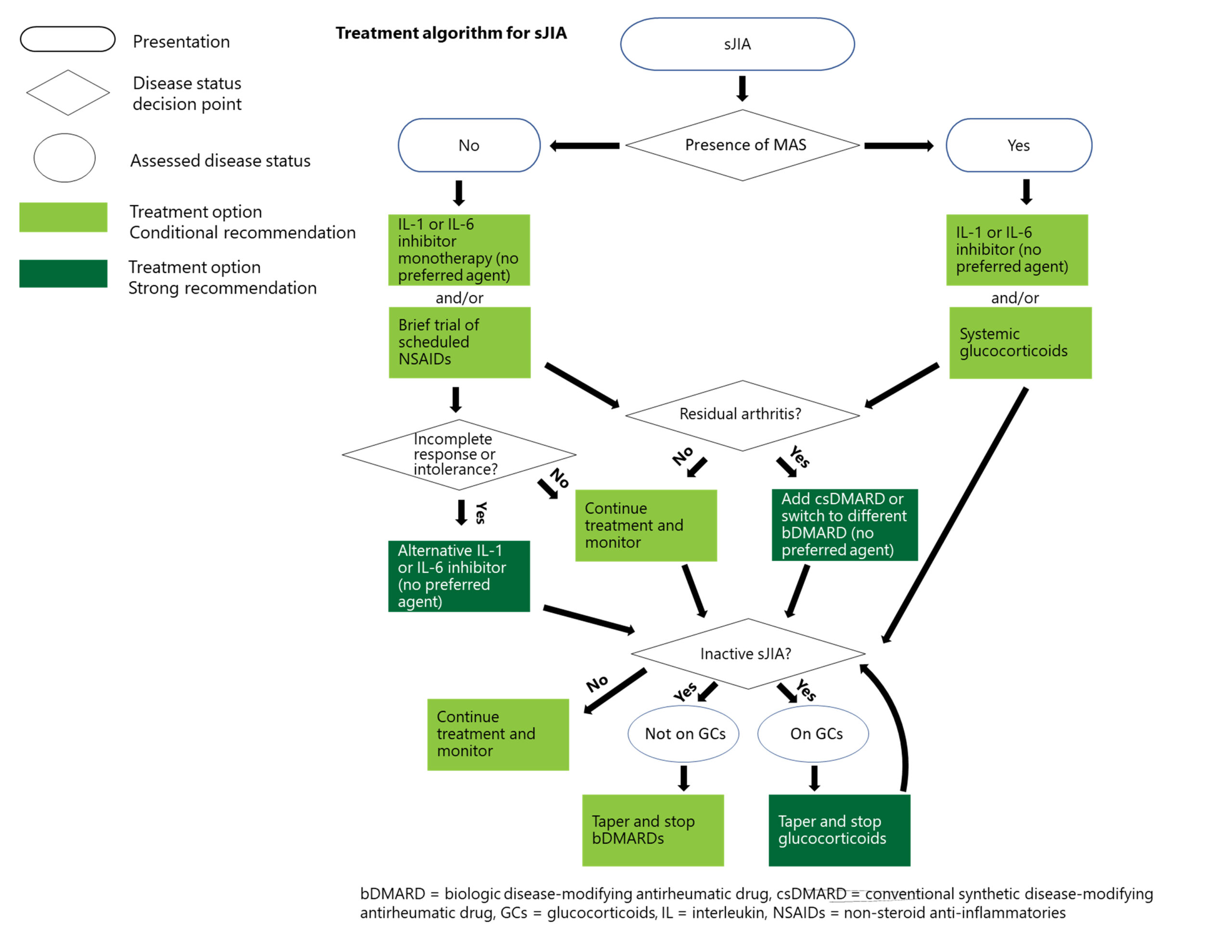
Treatment for Systemic JIA
Recommendations: For patients with systemic JIA without macrophage activation syndrome, IL-1 inhibitors, IL-6 inhibitors or NSAIDs are conditionally recommended as initial treatment, but with rapid escalation if response to NSAIDs is not fast and complete. Both oral glucocorticoids and csDMARDs are conditionally recommended against as initial therapy. IL-1 and IL-6 inhibitors are also strongly recommended over csDMARDs for treatment escalation.1
Recommendations for systemic JIA with macrophage activation syndrome are similar, but glucocorticoids are conditionally recommended as part of initial therapy, with tapering and discontinuation as soon as the disease is inactive.1
“When we say that the first-line treatment for systemic JIA is a biologic—not an NSAID or steroids or a conventional DMARD—that is really an extraordinary statement in terms of how much the field has moved,” Dr. Onel notes. “We have concerns with using biologics early in disease, but that has to be balanced with memories of the long-term outcomes for these children, which were terrible.”
With its internal organ involvement and severe, acute nature, systemic JIA requires more aggressive intervention compared to other forms of JIA—even more so when it is accompanied by macrophage activation syndrome.
“We wanted to make it clear that although we urge avoidance of daily steroids, basically all bets are off when there is also [macrophage activation syndrome],” Dr. Onel explains. “These children are extremely ill and need to be treated aggressively.”
Other Recommendations
Recommendations: All patients should receive baseline laboratory testing before treatment initiation for all medications. The guideline authors also provided guidance with respect to repeat testing for specific medications, such as screening with complete blood count and liver and kidney function tests every three to four months for patients taking methotrexate.2
Dr. Onel points out that this area is a good example of where shared decision making is important because families differ in how they perceive the burden and benefits of repeat blood tests. “You can’t tell how people will feel without asking,” she says. “We are trying to keep children safe without tormenting them by drawing their blood unnecessarily.”
Recommendations: Both inactivated and live virus type vaccines are strongly recommended for JIA patients who are not being treated with immunosuppressive drugs.
Inactivated vaccines are strongly recommended for patients being treated with immunosuppressive drugs; however, a conditional recommendation is that these patients not receive live virus vaccines, in accordance with Centers for Disease Control recommendations.2 Some evidence suggests that booster immunizations with live attenuated vaccines may be safe for children with JIA on specific immunosuppressants, but more work is needed to make a formal recommendation, Dr. Onel notes.
Thus, patients on immunosuppression not previously vaccinated need to rely on herd immunity for protection from measles and chicken pox, for example, rather than vaccination. Thus, it is imperative that family members and members of the household all receive appropriate immunizations.
Because the current vaccines for COVID-19 are inactivated vaccines, such vaccination is recommended for JIA patients regardless of whether they are on immunosuppression.
Recommendation: Screening for active synovitis or enthesitis using radiography prior to advanced imaging is strongly recommended against.2
Third-party payers often require clinicians to get an X-ray prior to a magnetic resonance imaging (MRI) scan, explains Dr. Cron. “But if you want to actually evaluate someone for inflammatory arthritis, X-rays tend not to be helpful in the short term. If you really want to know, particularly for the jaw, MRI is going to be much more helpful. [The X-ray] is unnecessary radiation for the child and a waste of time and money.”
Moving Forward
New data and treatment choices will be reflected in future versions of the guidelines. For example, the TNF inhibitor golimumab and the Janus kinase (JAK) inhibitor tofacitinib were both approved in 2020, after work on the guideline had already begun, and the IL-17 inhibitor secukinumab was approved just as the paper was being submitted. Janssen also has submitted an application to the U.S. Food & Drug Administration to expand the use of the IL-12/IL-23 inhibitor ustekinumab to treat juvenile psoriatic arthritis.8
“I think IL-17 blockade will likely become part of the armamentarium for kids with JIA.” says Dr. Cron. “It’s already getting studied pretty well on the adult side. JAK inhibitors will get more attention for pediatric arthritis as well, and IL-12/23 blockers may be of utility.”
Both Dr. Onel and Dr. Cron would like to see more information comparing different biologics in JIA and more information about which one might make sense for different patients. Ideally, we could work toward more of a personalized medicine approach and identify indicators via genetics, lab work and clinical signs about the best approaches for different patients.
“We also need to study the things that patients really want to know the answers to as well, like the potential impact of different diets,” says Dr. Onel. “That means working with families in our research group in the [Childhood Arthritis and Rheumatology Research Alliance].”
Dr. Cron would also like to see studies analyzing whether initial treatment via bDMARDs would be beneficial not just in systemic JIA, but also in other JIA contexts.
“It’s a really emotionally laden illness, requiring injectable medications and lab tests, that affects young kids. We always are going to need to work as hard as we can to be as safe as we can while being as thoughtful as possible about impacts to quality of life,” concludes Dr. Onel.
Ruth Jessen Hickman, MD, is a graduate of the Indiana University School of Medicine. She is a freelance medical and science writer living in Bloomington, Ind.
References
- Onel KB, Horton DB, Lovell DJ, et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: Therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2022 Apr;74(4):521–537.
- Onel KB, Horton DB, Lovell DJ, et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: Recommendations for nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Arthritis Care Res (Hoboken). 2022 Apr;74(4):505–520.
- Beukelman T, Patkar NM, Saag KG, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: Initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;63(4):465–482.
- Ringold S, Weiss PF, Beukelman T, et al; American College of Rheumatology. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: Recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 2013 Oct;65(10):2499–2512.
- Ringold S, Angeles-Han ST, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: Therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res (Hoboken). 2019 Jun;71(6):717–734.
- Angeles-Han ST, Ringold S, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis–associated uveitis. Arthritis Care Res. 2019 Jun;71(6):703–716.
- van Mater H, Balevic SJ, Freed GL, Clark SJ. Prescribing for children with rheumatic disease: Perceived treatment approaches between pediatric and adult rheumatologists. Arthritis Care Res (Hoboken). 2018 Feb;70(2):268–274.
- Johnson & Johnson. Janssen submits application seeking U.S. FDA approval of Stelara (ustekinumab) for the treatment of pediatric patients with juvenile psoriatic arthritis. 2021 Oct 8.