 Type I interferons (IFNs) “are important to the initiation and maintenance of cutaneous lupus erythematosus and dermatomyositis,” says Victoria Werth, MD, a professor of dermatology at the Hospital of the University of Pennsylvania and the Corporal Michael J. Crescenz Department of Veterans Affairs Medical Center, both in Philadelphia.
Type I interferons (IFNs) “are important to the initiation and maintenance of cutaneous lupus erythematosus and dermatomyositis,” says Victoria Werth, MD, a professor of dermatology at the Hospital of the University of Pennsylvania and the Corporal Michael J. Crescenz Department of Veterans Affairs Medical Center, both in Philadelphia.
Dr. Werth is the co-author of a review that is part of a series on immunology for rheumatologists launched earlier this year in Arthritis & Rheumatology (A&R).1 In this new installment, Dr. Werth and co-author Grace A. Hile, MD, a dermatologist at the University of Michigan School of Medicine, Ann Arbor, review the role of type I IFN in the immunopathogenesis of cutaneous lupus erythematosus and dermatomyositis skin inflammation. They also discuss how emerging IFN-targeted therapies for systemic lupus erythematosus (SLE) could potentially be used to treat dermatomyositis skin disease.2
Disease Underpinnings
Although cutaneous lupus erythematosus and dermatomyositis “share common triggers, the type I IFN subtype and efficacy of current therapeutics differ and may indicate key differences in disease pathogenesis: cutaneous lupus erythematosus being more IFNα driven, whereas IFNβ plays a dominant role in dermatomyositis,” Dr. Werth explains. “In cutaneous lupus erythematosus and dermatomyositis the heterogeneity of response to treatment correlates with different inflammatory cells and cytokines in the skin, while the predominant dendritic cell in skin is often different in cutaneous lupus erythematosus relative to dermatomyositis.”

Victoria Werth, MD
The authors introduce the review with a clinical case study. Next, Dr. Werth and Dr. Hile provide an overview of type I IFNs and discuss triggers of skin disease, including ultraviolet light, medications, supplements and viral infections. The authors examine the role of IFNs in the immunopathogenesis of cutaneous lupus erythematosus and dermatomyositis. They finish with a review of the emerging therapies targeting type I IFNs.
The review includes detailed images and legends, an informative table and many references.
“There are validated end points for skin disease activity in cutaneous lupus and dermatomyositis that correlate with the quality of life in patients and with disease biomarkers. This has facilitated clinical and translational studies of therapeutic antibodies that target specific cytokines, pathways, or inflammatory cells that contribute to the inflammatory cascade seen in cutaneous lupus erythematosus and dermatomyositis,” Dr. Werth says. “These therapeutic approaches are discussed in this review. Understanding the pathogenesis of skin diseases and drivers that initiate disease, particularly the role of type I IFN, is key to the ongoing novel therapies under evaluation.”
Clinical Case
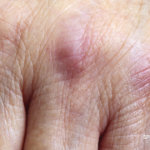
The clinical case follows a 29-year-old woman presenting with a six-month history of a pruritic rash that began after a viral illness. The patient’s symptoms include fatigue, hair loss and severe scalp pruritus. She was previously diagnosed with eczema, which did not improve on treatment with antihistamines and topical corticosteroids.
Clinical examination revealed violaceous periorbital edema, scaly red papules on the metacarpophalangeal joints of both hands, dilated capillaries of the proximal nail folds, scaly plaques on both elbows, and excoriated red plaques on her upper chest, back and hips. Antibody testing proved negative. The patient did not report any muscle weakness, strength and creatinine phosphokinase testing appeared normal, and magnetic resonance imaging of the thigh ruled out inflammation or edema.
The patient was diagnosed with clinically amyopathic dermatomyositis and treated with methotrexate, but this did not improve her skin and led to debilitating intractable pruritus. Mycophenolate mofetil treatment provided minimal improvement. However, given the lack of muscle involvement, the patient’s health insurance did not approve using intravenous immunoglobulin (IVIG).
The authors note that patients with clinically amyopathic dermatomyositis comprise up to 60% of dermatomyositis cases in dermatology clinics. As in the clinical case, “skin disease is often refractory to current systemic directed therapies and results in debilitating pruritus and increased morbidity,” the authors write. “Given the effectiveness of IVIG, and the likelihood of success with IVIG in this case, it is essential that dermatomyositis trials include amyopathic dermatomyositis patients so they can access appropriate therapies.”
Cutaneous Lupus Erythematosus vs. Dermatomyositis
Knowledge remains limited regarding both the pathogenesis of dermatomyositis and the drivers of skin disease.
The clinical case “raises several important issues related to the immunologic underpinnings of cutaneous lupus erythematosus and dermatomyositis,” including the roles of type I IFN in triggering skin disease and IFN in the pathogenesis of skin inflammation, the authors write. “Can we apply what we know about IFN-targeted therapeutics in cutaneous lupus erythematosus and dermatomyositis to develop better treatments for skin disease?”
Cutaneous lupus erythematosus and dermatomyositis remain challenging to differentiate, especially if the disease is limited to the skin, with rashes appearing similarly in both conditions. In dermatomyositis, as with cutaneous lupus erythematosus, type I IFNs appear highly upregulated in the blood, muscle and skin, correlating to disease activity. Further, in genetically predisposed individuals, cutaneous lupus erythematosus and dermatomyositis skin are likely induced or triggered by increased IFN exposure, as with sunlight, medications such as immune checkpoint inhibitors and hydroxyurea, immune-stimulating supplements or viral illnesses, including COVID-19.
The review includes an image depicting the similarities and differences between cutaneous lupus erythematosus and dermatomyositis.
Potential Therapies
No therapies approved by the U.S. Food & Drug Administration exist for the treatment of cutaneous lupus erythematosus or skin disease in dermatomyositis.
Several monoclonal antibodies and small molecule inhibitors to target the type I IFN signaling pathway are under development. These “promising new avenues for treatment” include therapies targeting IFNα or IFNβ, interferon-α/β receptor alpha chain (IFNAR), plasmacytoid dendritic cells, toll-like receptors (TLRs) and their downstream IFN signaling pathways, including Janus kinase (JAK) inhibitors and tyrosine kinase 2 (TYK2) inhibitors.
The authors also discuss the cannabinoid receptor type 2 agonist lenabasum, noting a clinical trial of its efficacy and safety. “The degree and consistency of clinical benefit, combined with a favorable safety profile, warrant further evaluation of lenabasum for skin predominant dermatomyositis,” they write.
A detailed figure summarizes the type I IFN signaling pathway, disease triggers and mechanisms of new therapeutics, while a table incorporates the emerging therapies in cutaneous lupus erythematosus and dermatomyositis.
“Skin only, skin predominant and post-myopathic skin disease in dermatomyositis is more common than previously reported,” Dr. Werth concludes. “Clinicians play an important role in advising patients of the immunostimulatory properties of several herbs that show differential activation of immune cells in dermatomyositis relative to normal controls. Current and ongoing trial data mostly require active myositis, excluding patients with predominantly skin disease, and this often limits the ability to get access to medications covered for those with muscle disease that would also work for the skin findings of dermatomyositis.”
Katie Robinson is a medical writer based in New York.
References
- Bucala R, Solomon DH. Immunology for the rheumatologist: Arthritis & Rheumatology introduces a new problem-based immunology review series with great educational potential. Arthritis Rheumatol. 2024 Jan;76(1):9–10.
- Hile GA, Werth VP. Understanding the role of type I IFN in cutaneous lupus and dermatomyositis: Towards better therapeutics. Arthritis Rheumatol. 2024 Sep 11.