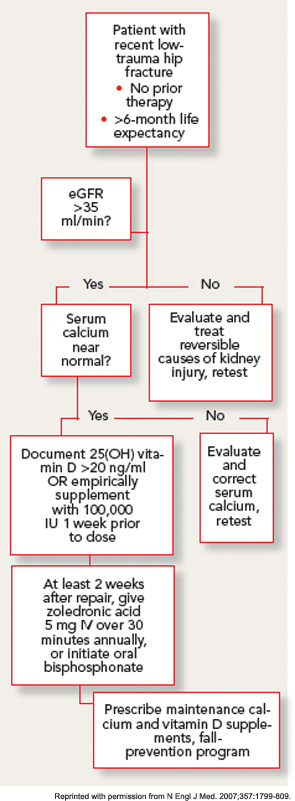
With this approach, no clinically significant cases of hypocalcemia were observed, and laboratory-detected hypocalcemia was quite rare and similar in both groups. No cases of vitamin D toxicity were found. Thus, for patients with normal calcium levels, an approach of universal repletion prior to bisphosphonate initiation appears safe. However, it is prudent to await further test results in patients with abnormal corrected serum calcium levels. While the serum levels of 25(OH) vitamin D at which bisphosphonates are safe has not been clearly determined in clinical studies, expert opinion holds that patients with serum levels above 20 ng/mL are unlikely to develop significant hypocalcemia.
GFR declines in most people with advancing age, and the prevalence of renal impairment is high in patients with hip fracture. Although most of the circulating serum bisphosphonate is deposited rapidly in the bone, nephrotoxicity has been documented with intravenous preparations, particularly in oncology populations receiving high dose therapy.16 Therefore, the label for oral and intravenous bisphosphonates excludes patients with GFR less than 30–35 mL/min, and renal safety has been scrutinized carefully with newer agents. No cases of clinically apparent acute kidney injury were observed in the zoledronic acid osteoporosis trials. Prospective laboratory studies in patients undergoing intravenous zoledronic acid therapy demonstrate a small acute decrease in GFR, which rapidly resolves, and no appreciable difference in renal function at one year.17 The infusion rate appears to be an important determinant of renal toxicity, thus minimum infusion times of 30 minutes have been recommended. It is prudent in older populations to document adequate hydration and baseline renal function with physical examination and laboratory testing prior to starting a bisphosphonate.
Patients in the post–hip fracture period usually have new mobility impairments and pain, and may be transitioning to a variety of healthcare settings over a short period. Therefore, minimizing the number of outpatient visits required to initiate osteoporosis treatment is an important goal. Although it would seem to be attractive to give the first dose of zoledronic acid during the hospitalization for hip-fracture surgery, there are several reasons that this is generally not practical. First, ensuring vitamin D sufficiency through laboratory testing or empiric repletion frequently takes longer than the average length of stay after hip fracture. In addition, post-hoc analysis of the HORIZON-RFT data strongly suggests that zoledronic acid has significantly lower efficacy on both BMD and fracture endpoints when given in the first two weeks after fracture.18This effect may occur because of the avid, preferential uptake of the drug at the fracture site, leaving less available for the rest of the skeleton.


