ACR CONVERGENCE 2021—Moderated by Dan Mandel, MD, a rheumatologist and associate clinical professor of medicine, the University of California, Irvine, the session Update on Extrapulmonary Sarcoidosis offered participants a multidisciplinary review of the features and management of systemic sarcoidosis.
Neurology
Jeffrey M. Gelfand, MD, associate professor of clinical neurology at the University of California, San Francisco, gave the first presentation, which he summarized as “what neurologists want rheumatologists to know about recognizing and treating neurosarcoidosis.”
He explained that although neurosarcoidosis was traditionally believed to affect approximately 5–10% of all sarcoidosis patients, a more recent series revealed that as many as 15–25% of sarcoidosis patients had evidence of neurosarcoidosis.1 Whatever the actual incidence, Dr. Gelfand emphasized that neurologic involvement increases the risk of morbidity and mortality in patients with sarcoidosis.
“A key clinical teaching point,” said Dr. Gelfand, “is that most—approximately 50–70%, of neurosarcoidosis patients—particularly patients with central nervous system neurosarcoidosis, present with the neurologic syndrome.”1 This neurosarcoidosis presentation then leads to a sarcoidosis diagnosis. According to Dr. Gelfand, in approximately 10–20% of patients, neurosarcoidosis may also occur as isolated to the nervous system. “So although isolated neurosarcoidosis is rare, it’s not that rare,” he emphasized.
In 2018, the Neurosarcoidosis Consortium updated the consensus diagnostic criteria for neurosarcoidosis.2 The bottom line, according to Dr. Gelfand, is that a diagnosis of neurosarcoidosis is made when the clinical presentation and diagnostic evaluation are consistent with neurosarcoidosis with a clinical syndrome typical of granulomatous invasion of the nervous system.
Patients should have pathological evidence of sarcoidosis in the central nervous system or peripheral nervous system for a definite diagnosis. If the patient has pathologic evidence of sarcoidosis elsewhere in their body and a consistent clinical syndrome, they are given a diagnosis of probable neurosarcoidosis.
Absent pathologic confirmation of granulomatous disease, the patient is given a diagnosis of possible neurosarcoidosis. “In many cases probable neurosarcoidosis is as close as we can reasonably get,” said Dr. Gelfand, “for example, in patients with spinal cord or brainstem disease.”
In all cases—definite, probable and possible neurosarcoidosis—diagnosis requires rigorous exclusion of other causes, particularly infection and malignancy. “It is this rigorous exclusion of other causes that keeps me up at night as a clinician,” said Dr. Gelfand.
In all cases—definite, probable & possible neurosarcoidosis—diagnosis requires rigorous exclusion of other causes, particularly infection & malignancy.
According to Dr. Gelfand, neurosarcoidosis commonly presents with cranial neuropathy, especially of the optic nerve, facial nerve and vestibulocochlear nerve. Although rarer than cranial neuropathy, leptomeningitis and pachymeningitis are also common phenotypes of neurosarcoidosis.
“Hydrocephalus is an important complication, often of chronic meningitis in neurosarcoidosis,” said Dr. Gelfand, noting that it often occurs with leptomeningitis and may require a shunt. “Stroke from, and due to, sarcoidosis as opposed to occurring co-morbidly in someone with sarcoidosis is rare, but can also occur,” concluded Dr. Gelfand.
Dr. Gelfand then discussed advances in treatment for neurosarcoidosis, including data supporting tumor necrosis factor (TNF) inhibitors for neurosarcoidosis. He ended his presentation by underscoring the association between TNF inhibitor exposure and inflammatory central nervous system events. For example, according to Dr. Gelfand, TNF inhibitors are associated with an increased risk of both demyelinating and non-demyelinating central nervous system events. Given what is known about the risk of harm, Dr. Gelfand therefore suggested avoiding TNF inhibitors in patients with a diagnosis of multiple sclerosis.
Patients who develop new, concerning neurological symptoms while receiving treatment with TNF inhibitors, should be evaluated in a timely manner, with consideration of neuroinflammation, as well as infectious and neurological diagnoses that may be unrelated to treatment.
Dermatology
Anthony P. Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic presented an update on dermatologic manifestations of sarcoidosis. He described the current understanding of the clinical features of patients with cutaneous sarcoidosis, discussed progress in the objective clinical assessment of sarcoidosis, and reviewed current and emerging treatment strategies for cutaneous sarcoidosis.
According to Dr. Fernandez, most patients have cutaneous manifestations at the time of diagnosis of systemic sarcoidosis and erythema nodosum may be the first presenting symptom of systemic sarcoidosis. Although the incidence of cutaneous sarcoidosis is not well defined, “what we do know,” said Dr. Fernandez, “is that African Americans have the highest rates of sarcoidosis in the U.S. and are more likely to have chronic cutaneous sarcoidosis than white Americans.”
Women also develop cutaneous disease more often than men.3
Dr. Fernandez also explained that an increasing number of medications is reported to induce cutaneous sarcoidosis and sarcoid-like granulomatous eruptions. Among such medications are interferon-α (IFN-α), immune checkpoint inhibitors, targeted kinase inhibitors and TNF inhibitors.4
According to Dr. Fernandez, although sarcoidosis may have different cutaneous presentations, the most common forms include papules, plaques, papulonodules, lupus pernio and subcutaneous nodules. Less common presentations are ichthyosiform, atrophic and ulcerative lesions, and mucosal lesions.
Dr. Fernandez suggested that rheumatologists use validated objective scoring tools for cutaneous involvement, such as the Cutaneous Sarcoidosis Activity and Morphology Instrument (CSAMI) and Sarcoidosis Activity and Severity Index (SASI). According to Dr. Fernandez, these tools can be useful in real-world clinical settings to follow progression or improvement of cutaneous disease by various specialists who treat sarcoidosis patients.
“Although local therapies are excellent when patients have mild disease,” said Dr. Fernandez, “Hydroxychloroquine, methotrexate and TNF inhibitors are effective for patients requiring systemic treatment.” He also pointed out that JAK inhibitors are emerging as a treatment option and described a recent case series of five patients with refractory cutaneous sarcoidosis who were successfully treated with the JAK inhibitor, tofacitinib.5
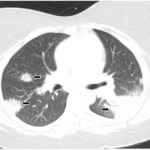
The patients in the case series had a mean treatment duration of 6.4 months and all five patients achieved CSAMI minimal clinically important differences with tofacitinib, and two achieved complete response at eight months. Additionally, three patients with pulmonary sarcoidosis reported cough reduction and improved exercise tolerance. Lastly, serial high-resolution computed tomography imaging in one patient showed progressive resolution of radiologic changes.
Lara C. Pullen, PhD, is a medical writer based in the Chicago area.
References
- MJ Bradshaw, Pawate S, Koth LL, et al. Neurosarcoidosis pathophysiology, diagnosis, and treatment. Neurol Neuroimmunol Neuroinflamm. 2021 Oct 4;8(6):e1084.
- Stern BJ, Royal W III, Gelfand JM, et al. Definition and consensus diagnostic criteria for neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018 Dec 1;75(12):1546–1553.
- Schupp JC, Freitag-Wolf S, Bargagli E, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J. 2018 Jan 25;51(1):1700991.
- Trilleras-Gomez AP, Hull KJ, Drew DZ. Case report and review of 7 similar cases in the literature: Cutaneous sarcoidosis as side effect of pembrolizumab plus chemotherapy in stage IV squamous cell carcinoma of lung. J Immunother. 2021 Feb–Mar 1;44(2):90–94.
- Kerkemeyer KL, Meah N, Sinclair RD. Tofacitinib for cutaneous and pulmonary sarcoidosis: A case series. J Am Acad Dermatol. 2021 Feb;84(2):581–583.